Molarity Made Easy: How to Calculate Molarity and Make Solutions
TLDRThis video from Ketzbook introduces viewers to the concept of molarity, a measure of solution concentration based on the number of solute particles dissolved. The presenter explains the molarity formula (M = n/V, where M is molarity, n is moles of solute, and V is the volume in liters) and guides through sample problems to calculate molarity, moles, and volume of solutions. The video also covers how to prepare a specific molarity solution, using copper(II) sulfate as an example. It includes a step-by-step demonstration on converting moles to grams using molar mass, and the practical process of making up a solution in a lab, emphasizing the importance of accurate measurement and technique.
Takeaways
- 🧪 Molarity is a concentration measurement that uses moles to express the number of solute particles dissolved in a solution.
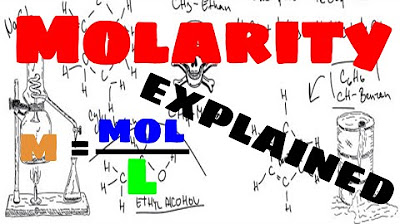
- 📏 The formula for molarity is M = n/V, where M is molarity, n is the number of moles of solute, and V is the volume of the solution in liters.
- 🔢 To calculate molarity, ensure that the volume is in liters, not milliliters, and use the appropriate conversion factors if necessary.
- ⚖️ When solving for moles (n), use the rearranged formula n = M * V, which helps isolate the number of moles needed.
- 🏗️ To find the volume (V) required for a given number of moles (n), use the rearranged formula V = n / M.
- 📦 When making a solution of a specific molarity, first calculate the number of moles needed, then convert moles to grams using the molar mass.
- 💧 For preparing a solution, use a volumetric flask and add the calculated amount of solute followed by distilled water up to the calibration mark.
- ➗ The molar mass of a compound is the sum of the molar masses of its constituent elements, which can be found on a chemical label or calculated if necessary.
- 🌡️ Solutions tend to dissolve more slowly if the solute is not in a fine powder form, so patience may be required during preparation.
- 📉 To convert between different units of volume (e.g., milliliters to liters), move the decimal point according to the metric prefix rules.
- 🔗 Understanding the concept of molarity is crucial for performing accurate chemical calculations and preparing solutions in a laboratory setting.
- 📝 Practice with sample problems helps solidify the understanding of molarity calculations and their application in real-world scenarios.
Q & A
What is the definition of molarity?
-Molarity is a concentration measurement that uses moles to measure how concentrated a solution is based on the number of solute particles dissolved.
What is the formula for calculating molarity?
-The formula for molarity is M = n / V, where M is molarity, n is the number of moles of solute, and V is the volume of the solution in liters.
How do you convert milliliters to liters?
-To convert milliliters to liters, you can multiply the volume in milliliters by a conversion factor fraction where one liter is equal to 1000 milliliters.
What is the molarity of a 125 mL solution containing 0.05 moles of hydrochloric acid?
-The molarity is calculated as 0.40 moles per liter (M), using the formula M = n / V and converting the volume from mL to L.
How many moles of sodium hydroxide are in 38 mL of a 0.5 mol/L solution?
-To find the moles (n), you use the equation n = M * V. For 38 mL of a 0.5 mol/L solution, n equals 0.5 mol/L times 0.038 liters, which is 0.019 moles.
How much 2.5 mol/L sulfuric acid should be used to get 0.12 moles of sulfuric acid?
-To find the volume (V) needed, use the equation V = n / M. For 0.12 moles of sulfuric acid at 2.5 mol/L, V equals 0.12 moles divided by 2.5 mol/L, which is 0.048 liters or 48 mL.
How would you make a 100 mL solution of a 0.4 molar solution of copper(II) sulfate?
-First, calculate the number of moles (n) needed using the formula n = M * V. For a 0.4 M solution and 100 mL (0.1 L), n equals 0.4 mol/L times 0.1 L, which is 0.04 moles. Then, convert moles to grams using the molar mass of copper(II) sulfate pentahydrate and weigh out the required amount.
What is the molar mass of copper(II) sulfate pentahydrate?
-The molar mass is calculated by adding the molar masses of copper (63.55 g/mol), sulfur (32.07 g/mol), oxygen (9 * 16 g/mol = 144 g/mol), and hydrogen (10 * 1.008 g/mol = 10.08 g/mol), resulting in a total molar mass of 249.7 g/mol.
How do you dissolve copper(II) sulfate pentahydrate in a volumetric flask to make a solution?
-Weigh out the required amount of copper(II) sulfate pentahydrate, add it to a 100 mL volumetric flask, and then add distilled water up to the fill line. Seal the flask with a stopper and gently mix the solution by inverting it several times to ensure complete dissolution.
What is the final step in making a copper(II) sulfate solution?
-The final step is to ensure the solution is well mixed. This can be done by inverting the flask with the stopper in place several times, allowing the copper(II) sulfate to dissolve completely, resulting in a homogeneous blue solution.
Why does the video recommend letting the copper(II) sulfate dissolve on its own?
-Copper(II) sulfate tends to dissolve slowly, especially if it is not in a fine powder form. Allowing it to dissolve on its own can prevent the need for excessive stirring and can be more effective in achieving a homogeneous solution.
What is the significance of the charge on copper in copper(II) sulfate?
-The charge on copper (+2) indicates that copper has lost two electrons and is in a +2 oxidation state. This is important for understanding the compound's chemical properties and reactivity, as well as for the stoichiometry in chemical reactions.
Outlines
🔍 Understanding Molarity and Its Calculation
This paragraph introduces molarity, a concentration measurement in chemistry that uses moles to indicate the number of solute particles dissolved in a solution. The formula for molarity is given as M = n/V, where M is molarity, n is the number of moles of solute, and V is the volume of the solution in liters. The paragraph demonstrates how to calculate molarity with an example problem involving a 125 mL solution with 0.05 moles of hydrochloric acid. It emphasizes the importance of converting units from milliliters to liters and concludes with the molarity result of 0.40 moles per liter.
🧪 Further Molarity Calculations and Preparing Solutions
The second paragraph delves into additional molarity problems, including calculating the number of moles in a given volume of sodium hydroxide solution and determining the volume of sulfuric acid needed for a specific number of moles. It also discusses how to prepare a solution of a certain molarity, exemplified by creating a 100 mL of a 0.4 molar solution of copper(II) sulfate. The process involves calculating the number of moles required, converting moles to grams using the molar mass, and then preparing the solution in a volumetric flask. The paragraph provides a step-by-step guide on measuring, dissolving, and mixing the solute to achieve the desired molarity.
Mindmap
Keywords
💡Molarity
💡Mole
💡Solute
💡Solvent
💡Volumetric Flask
💡Conversion Factor
💡Molar Mass
💡Pentahydrate
💡Concentration
💡Distilled Water
💡Metric Prefixes
Highlights
Molarity is a concentration measurement that uses moles to determine the concentration of a solute in a solution.
The formula for molarity is M = n/V, where M is molarity, n is the number of moles of solute, and V is the volume of the solution in liters.
To convert mL to liters, multiply by 0.001 or move the decimal point three places to the left.
The molarity of a 125 mL solution with 0.05 moles of hydrochloric acid is calculated to be 0.40 moles per liter.
When calculating moles, use the rearranged formula n = M * V.
For calculating the volume needed for a specific number of moles, use the formula V = n / M.
To prepare a solution of a given molarity, first calculate the number of moles required using the formula n = M * V.
The molar mass of copper(II) sulfate pentahydrate is calculated by adding the molar masses of its constituent elements.
Copper(II) sulfate pentahydrate is often used in labs due to its water of crystallization, which affects the molar mass.
The molar mass of copper(II) sulfate pentahydrate is 249.7 g/mol, used to convert moles to grams for solution preparation.
To make a 100 mL, 0.4 molar solution of copper(II) sulfate, weigh out 9.988 grams of the pentahydrate.
Use a 100 mL volumetric flask to prepare the solution, adding the weighed copper(II) sulfate pentahydrate first.
Add distilled water to the volumetric flask until it reaches the fill line, ensuring the solution is well mixed.
Copper(II) sulfate dissolves slowly, so it may be necessary to let it dissolve on its own to form a homogeneous solution.
The final solution of copper(II) sulfate will have a characteristic blue color.
The video provides a step-by-step guide on molarity calculations and solution preparation, suitable for educational purposes.
Ketzbook offers additional resources and information on their website for further learning.
Transcripts
Browse More Related Video

Concentration and Molarity explained: what is it, how is it used + practice problems

Trick to Calculate Molarity | Molarity practice problems

Molarity Practice Problems
Molarity Explained

Difference between Molarity and Molality

How to Do Solution Stoichiometry Using Molarity as a Conversion Factor | How to Pass Chemistry
5.0 / 5 (0 votes)
Thanks for rating: