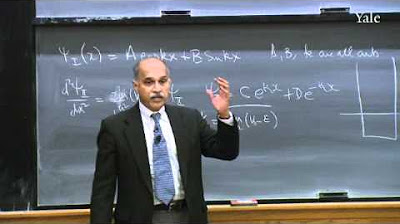25. Quantum Mechanics VII: Summary of postulates and special topics
TLDRThe transcript is a detailed lecture on quantum mechanics, focusing on the foundational postulates of the theory. The professor explains that quantum mechanics is not derived from mathematics but is based on experimental facts condensed into postulates. The lecture covers the concept of wave functions, which contain all the information about a particle, and how these functions are used to describe states of definite momentum and position. The professor also discusses the mathematical relationship between these states and the physical interpretation of the wave function's normalization. The lecture further explores the implications of the Schrödinger equation for the time evolution of quantum states and introduces the concept of stationary states. The Pauli Exclusion Principle and the behavior of identical particles, such as fermions and bosons, are also explained. The summary concludes with the impact of quantum mechanics on understanding atomic structure and the periodic table, emphasizing the importance of these principles in chemistry and physics.
Takeaways
- 📜 **Quantum Postulates**: Quantum mechanics is based on a set of postulates that encapsulate experimental facts rather than being purely mathematically derived.
- 🚀 **Wave Function**: The wave function (Y(x)) for a single particle in one dimension contains all the possible information about the particle, analogous to x and p in classical mechanics.
- 📏 **Normalization**: The wave function is always chosen to be normalized such that the integral of the absolute square of Y over all space is equal to 1.
- 🌟 **Momentum State**: There exists a wave function (Y_p(x)) that, when measured, guarantees a definite momentum for the particle, represented by e^(ipx/ℏ)/√L, where L is the length of the universe.
- ⚖️ **Quantization**: The momentum of a particle in a ring is quantized to (2πħ/L)n, where n is an integer, a result derived from mathematical considerations.
- 📍 **Position State**: A particle with a definite position (x_0) is described by a wave function that is a spike at x_0, or equivalently, obeys the equation xY_x0(x) = x_0Y_x0(x).
- 🔢 **Energy States**: States of definite energy are solutions to the Schrödinger equation and are important for understanding systems like atoms, which can only exist at certain energy levels.
- 🥳 **Superposition**: Quantum states can be superpositions of other states, leading to probabilities for different outcomes when measurements are made.
- 🕒 **Time Evolution**: The state of a quantum system changes with time according to the Schrödinger equation, which is a fundamental postulate of quantum mechanics.
- 🚫 **Pauli Exclusion Principle**: No two fermions can occupy the same quantum state simultaneously, a principle that has profound implications for the structure of atoms and the periodic table.
- ⚛️ **Identical Particles**: In quantum mechanics, identical particles are either bosons, which can occupy the same state, or fermions, which cannot, leading to different statistical behaviors.
Q & A
What is the fundamental principle behind the postulates of quantum mechanics?
-The postulates of quantum mechanics are based on experimental facts rather than mathematical deduction. They are designed to condense the understanding of quantum phenomena into a set of fundamental rules or assumptions that can be used to answer any question or solve any problem within the theory.
How does the wave function in quantum mechanics relate to the information about a particle?
-In quantum mechanics, the wave function (Y(x)) contains all the possible information about a single particle in one dimension. It is analogous to the position (x) and momentum (p) in classical mechanics and is normalized so that the integral of the wave function squared over all space is equal to 1.
What is the significance of the wave function Y_p(x) in describing a particle with definite momentum?
-The wave function Y_p(x) is a specific solution that guarantees, upon measurement, the particle will have a definite momentum (p). It is represented by the function e^(ipx/ℏ) divided by the square root of the length of the universe in which the particle exists.
How does the mathematical deduction of quantized momentum relate to the physical postulates in quantum mechanics?
-The mathematical deduction that momentum is quantized in the form of (2ph/L) times an integer (n) is separate from the physical postulates. It is a result that follows from the postulates, specifically the second postulate regarding the existence of a state with definite momentum.
What is the role of the Dirac delta function in describing a state of definite position in quantum mechanics?
-The Dirac delta function is suggested as a representation for a state of definite position (x_0), where the particle is guaranteed to be at x_0 upon measurement. However, the professor avoids using the technical term 'Dirac delta function' and instead describes it as a 'big spike at x_0' to illustrate the concept in a more accessible way.
How does the energy of a quantum state relate to the time-independent Schrödinger equation?
-The energy of a quantum state is found by solving the time-independent Schrödinger equation, which is a differential equation that relates the total energy of a system to the wave function of the system. The solutions to this equation give the allowed energy levels of the system.
What is the implication of the postulate regarding the probability of measuring a particular value of a physical variable in quantum mechanics?
-The postulate states that if a wave function Y(x) is expressed as a sum of different states Y_g(x), each multiplied by a coefficient A_g, the probability of measuring a particular value g is given by the square of the absolute value of A_g. This is determined by the integral of the product of the complex conjugate of Y_g(x) and Y(x).
What does the collapse of the wave function mean in the context of quantum measurement?
-The collapse of the wave function, as described by postulate 5, means that upon measurement of a physical variable (like position, momentum, or energy), the wave function reduces to a single term corresponding to the measured value. This implies that the act of measurement changes the state of the quantum system from one that is potentially many things to one that is only one specific thing.
How does the time evolution of a quantum state described by the Schrödinger equation ensure that certain states remain unchanged in their probability distribution over time?
-The Schrödinger equation describes how the wave function of a quantum system changes over time. For states of definite energy, known as stationary states, the probability distribution of the system remains unchanged over time. This is because the time-dependent phase factor in the wave function's evolution does not affect the probability distribution, which depends only on the absolute square of the wave function.
What is the significance of the Pauli Exclusion Principle in the context of the periodic table of elements?
-The Pauli Exclusion Principle states that no two fermions, such as electrons, can occupy the same quantum state simultaneously. This principle is fundamental to understanding the structure of atoms and the periodic table, as it dictates how electrons fill the available energy levels around the nucleus, leading to the observed periodicity in chemical properties.
How does the concept of identical particles in quantum mechanics lead to the classification of particles into bosons and fermions?
-In quantum mechanics, identical particles refer to particles that are indistinguishable from one another. The behavior of their joint wave function under the exchange of particle labels leads to the classification into bosons (where the wave function remains the same) and fermions (where the wave function changes sign). This distinction is crucial for understanding the statistical mechanics of particle systems and the behavior of particles at the quantum level.
Outlines
📚 Introduction to Quantum Mechanics Postulates
The professor begins by emphasizing the importance of understanding the postulates of quantum mechanics. These rules, based on experimental facts rather than mathematical deduction, form the foundation of the theory. The first postulate introduces the concept of a wave function, denoted as Y(x), which contains all the possible information about a particle in one dimension. The wave function must be normalized so that the integral of Y squared over all space equals one. The second postulate discusses the existence of a wave function for a state of definite momentum, Y_p(x), which is a specific function ensuring that measuring the momentum of a particle in this state yields a definite value, p. The professor also touches on the mathematical aspect of quantization of momentum in a ring and introduces the concept of a state of definite position, Y_x0, characterized by a spike function at x0.
📐 Mathematical Forms and Postulates in Quantum Mechanics
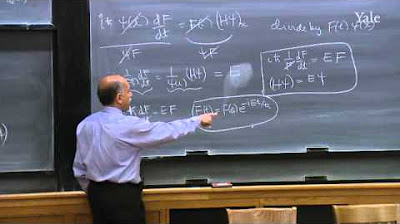
The discussion continues with the exploration of special mathematical forms in quantum mechanics. The professor explains that taking the derivative of a function, Y(x), typically results in a new function, but in quantum mechanics, there are special functions that, when differentiated, result in a multiple of the same function. This leads to the concept of a state of definite position, described by a spike function at x0, which is unique because multiplying by x affects only the non-zero value at x0. The professor also introduces the idea that states of definite energy, E, are solutions to a specific equation involving the Hamiltonian operator and the potential, V(x). However, this is not elevated to a postulate due to its relation to the known states of definite momentum and position.
🚀 Postulates on Probability and State Collapse
The fourth postulate deals with the probability of measuring a specific value, g, such as momentum or energy, within a quantum state described by a wave function Y(x). The probability is given by the square of the coefficient, |A_g|^2, obtained from the integral of the product of the conjugate of Y_g and Y. The fifth postulate addresses the collapse of the wave function upon measurement: if a measurement yields a specific value, g_0, the wave function collapses to just that term, Y_g0. This postulate highlights the unique feature of quantum mechanics where measurement affects the state of the system. The professor also clarifies that states of definite momentum and position are not compatible, as measuring one affects the knowledge of the other.
⏲️ Time Evolution of Quantum States
The sixth postulate describes the time evolution of quantum states according to the Schrödinger equation, which is fundamental to quantum mechanics. The professor explains that if a system starts in a state of definite energy, the wave function will evolve in time by acquiring a phase factor, e^(iEt/ℏ). This results in stationary states, where the probability density remains time-independent. For arbitrary initial states, the system's state at a later time can be found by expanding the initial state in terms of energy eigenstates and applying the time evolution to each term. The professor assures students that the content following this point will not be on the exam, encouraging them to relax and gain an understanding of the broader applications of quantum mechanics.
⚛️ Quantum Mechanics and the Hydrogen Atom
The professor delves into the application of quantum mechanics to understand atoms, specifically the hydrogen atom. By solving the Schrödinger equation for the hydrogen atom, one can determine the allowed energy levels, which are quantized and described by the formula E = -13.6 eV / n^2, where n is an integer. These energy levels dictate the absorption and emission spectra of the hydrogen atom, which is how it is observed and studied. The professor humorously recounts the story of Schrödinger and the challenges he faced in solving the equation, highlighting the historical context of these scientific breakthroughs.
🌟 Absorption, Emission, and Quantum States
The explanation of how atoms, such as hydrogen, absorb and emit light is presented. The difference in energy between levels is given by the formula E_f - E_i = ℏw, which corresponds to the energy of a photon. The professor emphasizes that understanding the quantum states and their corresponding wave functions is crucial for calculating the rates of absorption and emission. The concept of degeneracy, where multiple states can exist at a given energy level, is introduced, and the role of electron spin in doubling the number of states is mentioned. The Pauli exclusion principle is highlighted as a fundamental principle that prevents two fermions from occupying the same quantum state, which has profound implications for the structure of atoms and the periodic table.
🤝 Identical Particles and Quantum States
The treatment of identical particles in quantum mechanics is discussed. The professor explains that for identical particles, such as electrons, which are fermions, the wave function must be antisymmetric, meaning it changes sign if the particles' coordinates are swapped. This leads to the Pauli exclusion principle, which states that no two fermions can occupy the same quantum state. In contrast, bosons, such as photons, can be in the same state and do not follow this restriction. The distinction between bosons and fermions is crucial for understanding the behavior of particles in various systems and is fundamental to the structure of the periodic table and the properties of atoms.
Mindmap
Keywords
💡Quantum Mechanics
💡Wave Function
💡Postulates
💡Normalization
💡Momentum
💡Schrödinger Equation
💡Pauli Exclusion Principle
💡Bosons and Fermions
💡Superposition
💡Entanglement
💡Energy Levels
Highlights
Quantum mechanics is a theory based on experimental postulates rather than pure mathematics.
The wave function Y(x) for a single particle in one dimension contains all possible information about the particle.
Normalization of the wave function is a convention that ensures the squared integral of Y is 1, representing the probability density.
A state of definite momentum is represented by the wave function Y_p(x) = e^(ipx/ℏ)/√L, where L is the length of the universe.
The quantization of momentum is a mathematical deduction that results in discrete values given by (2ph/L) * n, where n is an integer.
A state of definite position x_0 is described by a wave function that is a 'spike' at x_0, which is technically a Dirac delta function.
The act of measurement in quantum mechanics collapses the wave function to a single outcome, changing the system's state.
The Schrödinger equation governs the time evolution of quantum states and is a fundamental postulate of quantum mechanics.
Stationary states are solutions to the Schrödinger equation where the probability density is time-independent.
The energy levels of the hydrogen atom can be calculated using quantum mechanics, providing insight into atomic absorption and emission spectra.
The uncertainty principle in quantum mechanics states that the product of the uncertainties in position and momentum is greater than or equal to ℏ/2.
The Pauli Exclusion Principle, a consequence of quantum mechanics, states that no two fermions can occupy the same quantum state simultaneously.
The behavior of identical particles in quantum mechanics differs from classical mechanics, leading to the concept of bosons and fermions.
The Pauli Principle is fundamental to understanding the structure of the periodic table and the behavior of electrons in atoms.
Quantum mechanics provides a framework for understanding the properties of atoms, including their energy levels and chemical reactivity.
The wave function for a system of identical particles must be symmetric (bosons) or antisymmetric (fermions) under particle exchange.
The Pauli Principle ensures that atoms have distinct energy levels and that electrons fill these levels in a specific order, leading to the periodic table's structure.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: