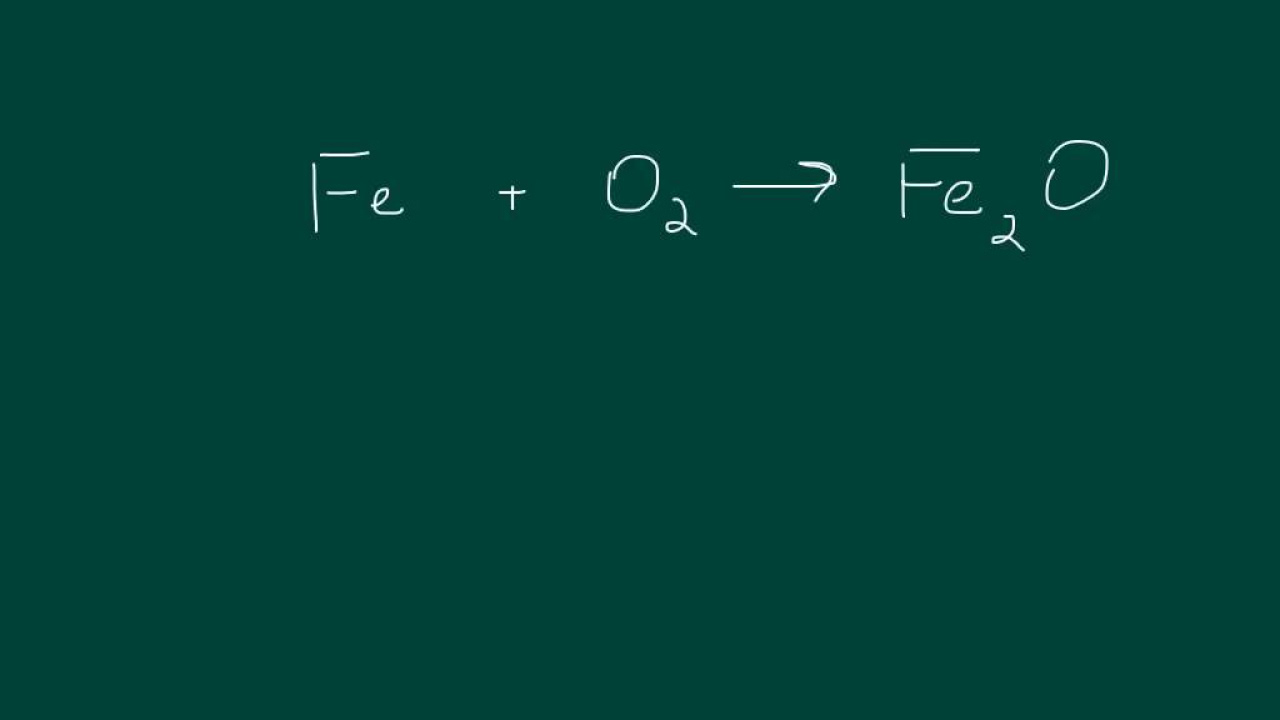Grade 9 Natural Science: Balancing Equations
TLDRThe video script is an educational lesson on chemical reactions, focusing on the process of balancing chemical equations. The instructor begins with a review of the previous lesson on chemical reactions and balanced equations, emphasizing the importance of understanding the molecular formulas for elements like hydrogen (H2) and oxygen (O2). The lesson then dives into practicing the writing and balancing of chemical equations using symbols from the periodic table. The instructor provides step-by-step guidance on how to balance equations for reactions such as hydrogen plus oxygen yielding water, magnesium plus oxygen yielding magnesium oxide, and hydrogen plus chlorine yielding hydrochloric acid. The principle of balancing is explained as ensuring that the total number and type of atoms on the reactant side equal those on the product side. The session concludes with a more complex example involving iron and oxygen, demonstrating the need to balance both the iron and oxygen atoms. The instructor uses this opportunity to highlight the importance of mathematical skills in chemistry and encourages students to apply these principles to balance more complex equations, promising to explore the reactions of metals with oxygen in the next session.
Takeaways
- 🔍 **Balancing Equations**: Chemical equations must be balanced to ensure the total number and type of atoms on the reactant side equal those on the product side.
- ⚖️ **Molecular Representation**: Elements like hydrogen (H2) and oxygen (O2) are diatomic and should be represented as such in chemical equations, not as single atoms.
- 💧 **Writing Water Formula**: The chemical formula for water is H2O, representing two hydrogen atoms bonded to one oxygen atom.
- 📜 **Balancing by Counting**: Balancing equations is akin to a counting game, where you ensure the number of each element is the same on both sides of the equation.
- 🔢 **Use of Coefficients**: Coefficients (whole numbers) are placed in front of chemical formulas to balance the equation.
- 🛠️ **Balancing Strategy**: Start by balancing elements that appear in different forms on either side of the equation, such as oxygen in O2 and O3.
- 🧲 **Diatomic Molecules**: Halogens like chlorine (Cl2) also exist as diatomic molecules, which must be considered when writing and balancing equations.
- 🚫 **Avoiding Shortcuts**: Do not assume that balancing one element will automatically balance the others; each must be addressed individually.
- 🧪 **Practical Application**: Balancing equations is a fundamental skill in chemistry, critical for understanding and performing chemical reactions accurately.
- 📈 **Complex Balancing**: Some equations may require multiplying entire formulas by coefficients to achieve balance, as demonstrated with iron oxide (Fe2O3).
- 🔬 **Continual Learning**: The process of balancing equations improves with practice, emphasizing the importance of repetition and application in learning chemistry.
Q & A
What was the last topic discussed before the current one?
-The last topic discussed was chemical reactions, specifically focusing on balanced equations.
Why is it important to balance chemical equations?
-Balancing chemical equations is important because it ensures that the total number and type of atoms of the reactants equal the same for the products, adhering to the law of conservation of mass.
What is the molecular formula for hydrogen gas?
-The molecular formula for hydrogen gas is H2, as hydrogen cannot exist on its own and reacts as a diatomic molecule.
What is the molecular formula for oxygen gas?
-The molecular formula for oxygen gas is O2, as oxygen also exists as a diatomic molecule in its standard state.
How do you write the chemical formula for water?
-The chemical formula for water is H2O, which indicates two hydrogen atoms bonded to one oxygen atom.
What is the process of balancing a chemical equation?
-Balancing a chemical equation involves adjusting the coefficients (the numbers in front of the chemical formulas) to ensure that the number of atoms for each element is the same on both sides of the equation.
What is the balanced equation for magnesium reacting with oxygen to form magnesium oxide?
-The balanced equation is 2Mg + O2 → 2MgO, which shows equal numbers of magnesium and oxygen atoms on both sides.
What is the chemical formula for hydrochloric acid?
-The chemical formula for hydrochloric acid is HCl, which is formed from the reaction of hydrogen (H2) and chlorine (Cl2).
How did the instructor approach balancing the equation for copper and oxygen to form copper oxide?
-The instructor first balanced the oxygen atoms by placing a coefficient of 2 in front of CuO. Then, to balance the copper atoms, a coefficient of 2 was placed in front of Cu on the reactant side.
What is the chemical formula for iron(III) oxide?
-The chemical formula for iron(III) oxide is Fe2O3, which indicates two iron atoms and three oxygen atoms.
What is the balanced equation for iron reacting with oxygen to form iron(III) oxide?
-The balanced equation is 4Fe + 3O2 → 2Fe2O3, ensuring that the number of iron and oxygen atoms are equal on both sides.
What is the next topic that will be covered after the discussion on balancing chemical equations?
-The next topic to be covered is the reactions of metals with oxygen.
Outlines
🔬 Introduction to Chemical Reactions and Balancing Equations
The video script begins with a recap of the previous lesson on chemical reactions, emphasizing the importance of balancing equations. The instructor guides viewers through the process of writing and balancing chemical equations using molecular formulas and periodic table symbols. The first example involves hydrogen, oxygen, and water, highlighting the need to use H2 and O2 to represent hydrogen and oxygen molecules, respectively. The concept of balancing is introduced as ensuring equality of elements on both sides of the equation, using a practical analogy of balancing buckets of water.
🧪 Balancing Equations: Magnesium, Hydrogen, and Chlorine Reactions
The script continues with the explanation of balancing chemical equations, emphasizing the necessity for the total number and type of atoms in reactants to equal those in products. The instructor demonstrates how to balance equations for reactions involving magnesium with oxygen to form magnesium oxide and hydrogen with chlorine to form hydrochloric acid. The process involves doubling molecules to achieve equality on both sides of the equation, ensuring that the reaction is chemically balanced.
📚 Advanced Balancing: Copper and Iron Oxides
The video script moves on to more complex examples, including the reaction of copper with oxygen to form copper oxide and iron with oxygen to form iron oxide. The instructor shares a personal technique for balancing equations, suggesting to address oxygen first due to its presence in both reactants and products. For copper oxide, placing a coefficient of 2 in front of the product balances the equation. For iron oxide, the process is more involved, requiring the doubling of the product side before balancing the reactants. The instructor stresses the importance of counting and applying mathematical skills to achieve a balanced equation.
🎓 Conclusion and Preview of Upcoming Topics
The script concludes with the instructor summarizing the process of balancing chemical equations, reiterating that it is essentially a counting game aimed at ensuring the same number of each element on both sides of the equation. The video ends with a preview of the next topic, which will focus on the reactions of metals with oxygen. The instructor encourages viewers to stay tuned for the next part of the series.
Mindmap
Keywords
💡Chemical Reactions
💡Balanced Equations
💡Molecular Formula
💡Periodic Table
💡Metals with Oxygen
💡Reactants and Products
💡Hydrogen (H2)
💡Oxygen (O2)
💡Magnesium Oxide
💡Hydrochloric Acid
💡Iron Oxide
Highlights
The lesson focuses on writing and balancing chemical equations using periodic table symbols.
Hydrogen and oxygen exist as diatomic molecules (H2 and O2) and cannot exist alone.
Water is represented as H2O, with two hydrogens bonded to one oxygen.
Balancing chemical equations means ensuring the total number and type of atoms on each side of the equation are equal.
The reason for balancing equations is that the total number of atoms of reactants must equal the total number of atoms of products.
Magnesium plus oxygen forms magnesium oxide, with a 2 in front of magnesium on both sides to balance the equation.
Hydrogen plus chlorine forms hydrochloric acid, with a 2 in front of HCl to balance the hydrogens and chlorines.
Balancing equations involves counting the number of each element on both sides and adjusting coefficients until they match.
Copper plus oxygen forms copper oxide, with a 2 in front of Cu and CuO to balance copper and oxygen.
Iron plus oxygen forms iron oxide, with a 4 in front of Fe and a 3 in front of O2 to balance iron and oxygen.
The instructor shares a technique of first balancing the oxygen in the equation, then adjusting other elements as needed.
For more complex equations, you may need to double the products on the right side first before balancing the reactants.
Balancing equations is a counting game of ensuring the same number of each element appears on both sides of the equation.
The instructor emphasizes the importance of knowing the periodic table to recognize elements in their molecular or chemical formulas.
The lesson includes examples of balancing equations for reactions involving hydrogen, oxygen, magnesium, chlorine, copper, and iron.
The instructor provides step-by-step guidance and explanations for writing and balancing each equation.
The lesson concludes with a preview of next week's topic on reactions of metals with oxygen.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: