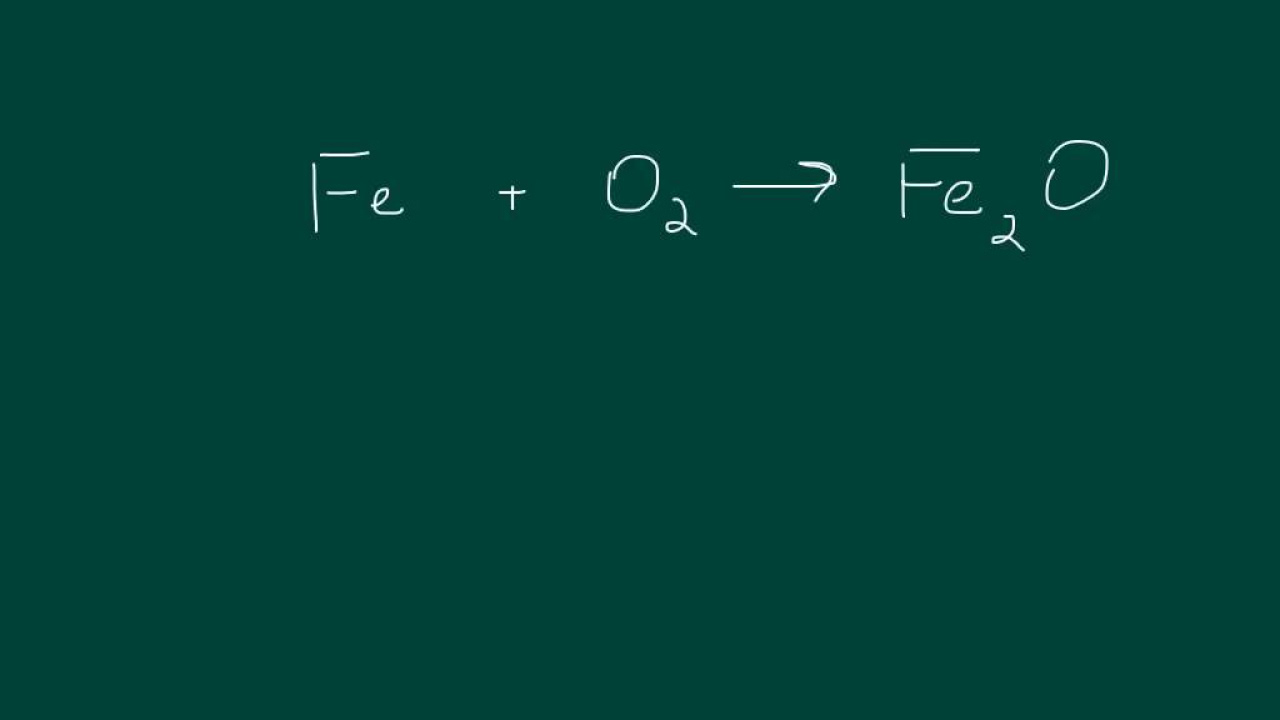How To Balance Chemical Equations
TLDRThe video script provides a comprehensive guide on how to balance chemical equations. It begins with a basic example of magnesium reacting with nitrogen gas to form magnesium nitride, emphasizing the importance of ensuring equal numbers of atoms on both sides of the equation. The script then delves into various types of chemical reactions, including combustion and single replacement reactions, offering step-by-step instructions on balancing each. It highlights the strategy of balancing elements or ions with the highest charge first and treating polyatomic ions as single units. The video also addresses common pitfalls, such as avoiding fractional coefficients and correcting mistakes during the balancing process. The presenter encourages practice with more complex examples and provides links for additional practice problems, making the content both informative and engaging for viewers interested in mastering chemical equation balancing.
Takeaways
- 🧪 To balance a chemical equation, ensure the number of each type of atom is equal on both sides of the equation.
- 🔍 Start by listing the number of atoms for each element on both sides of the equation.
- 📐 You can change coefficients but not subscripts when balancing chemical equations.
- 🔄 For balancing, sometimes it's beneficial to balance one element over another, but you can adjust until the equation is correct.
- ✅ In the case of combustion reactions, it's recommended to balance carbon atoms first, then hydrogen, and oxygen last.
- 🚫 Avoid using fractional or decimal coefficients; instead, use whole numbers by multiplying through by a common factor if needed.
- 🤝 In single replacement reactions, balance the number of atoms for each element to ensure equality on both sides.
- 🤔 For double replacement reactions with polyatomic ions, balance ions as a whole rather than individual elements.
- 🧠 Understanding the charges of ions can help in balancing reactions, especially when dealing with polyatomic ions.
- 📉 Start balancing with the ion that has the highest charge, which can simplify the process.
- 🔗 Balancing chemical equations is a systematic process that requires step-by-step adjustments until all atoms are accounted for equally on both sides.
- 📚 Practice is key for mastering the skill of balancing chemical equations, and there are resources available for additional practice.
Q & A
What is the primary goal of balancing a chemical equation?
-The primary goal of balancing a chemical equation is to ensure that the number of atoms for each element is the same on both sides of the equation, adhering to the law of conservation of mass.
Why is it important not to change the subscripts when balancing chemical equations?
-Changing the subscripts would alter the chemical identity of the compound, which is not allowed when balancing equations. Coefficients in front of compounds can be changed to balance the atoms, but the subscripts represent the actual chemical structure of the compound.
What is the recommended order for balancing atoms in a combustion reaction?
-For combustion reactions, it is recommended to balance carbon atoms first, then hydrogen atoms, and finally oxygen atoms last.
How do you address fractional coefficients when balancing chemical equations?
-Fractional coefficients are not preferred in chemistry. If you end up with a fraction, you multiply every coefficient in the equation by the denominator to convert it to a whole number.
What is a polyatomic ion, and how should it be treated when balancing chemical equations?
-A polyatomic ion is a group of two or more atoms that function as a single ion with a charge. It should be treated as a single unit when balancing chemical equations, rather than balancing the individual elements within it.
What is the least common multiple (LCM) and how is it used in balancing chemical equations?
-The least common multiple (LCM) is the smallest number that is a multiple of two or more numbers. It is used to balance the number of atoms of elements that appear in different quantities on each side of the equation by adjusting the coefficients.
Why is balancing the element with the highest charge often easier in double replacement reactions?
-Balancing the element with the highest charge can simplify the process because it often involves fewer adjustments to the other elements in the equation. This is particularly useful when dealing with polyatomic ions.
What is a single replacement reaction and how do you balance it?
-A single replacement reaction is a type of chemical reaction where an element in a compound is replaced by another element. To balance it, you adjust the coefficients in front of the compounds so that the number of atoms for each element is equal on both sides of the equation.
What is the role of the law of conservation of mass in chemical reactions?
-The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. This law is fundamental when balancing chemical equations, as it ensures that the total mass of reactants equals the total mass of products.
How do you balance a chemical equation involving a compound like magnesium nitride?
-To balance a chemical equation involving magnesium nitride, you would start by ensuring that the number of magnesium and nitrogen atoms is the same on both sides of the equation. This may involve adjusting the coefficients in front of magnesium and nitrogen compounds.
What is the process for balancing a double replacement reaction?
-For a double replacement reaction, you should balance the polyatomic ions or the ions with the highest charge first. Then, adjust the remaining ions to ensure that the number of each type of ion is balanced on both sides of the equation.
Why is it advised to multiply every term in the equation by the denominator when dealing with fractions?
-Multiplying every term in the equation by the denominator converts any fractional coefficients to whole numbers, which is the standard practice in chemistry to avoid ambiguity and to maintain the simplicity of the balanced equation.
Outlines
🔬 Balancing Chemical Equations: Magnesium and Nitrogen
This paragraph introduces the concept of balancing chemical equations using the example of magnesium reacting with nitrogen gas to form magnesium nitride. The key principle is ensuring that the number of atoms for each element is equal on both sides of the equation. The process involves listing the number of atoms on each side and then adjusting coefficients to achieve balance. The magnesium and nitrogen atoms are balanced by multiplying the magnesium coefficient by three, resulting in three magnesium atoms on both sides of the equation.
🧪 Balancing Equations: Nitrogen and Hydrogen to Ammonia
The second paragraph demonstrates the balancing of a chemical equation for the reaction between nitrogen gas and hydrogen gas to produce ammonia. The process involves balancing the nitrogen atoms first by multiplying the ammonia coefficient by two, and then balancing the hydrogen atoms by adjusting the hydrogen coefficient to six. The summary emphasizes the technique of balancing elements step by step and adjusting coefficients to ensure equality of atoms on both sides of the equation.
⚗️ Balancing Equations: Sulfur and Fluorine to Sulfur Hexafluoride
This paragraph discusses the balancing of a chemical equation for the reaction between elemental sulfur (S8) and fluorine gas to form sulfur hexafluoride. The approach involves balancing the fluorine atoms first, then adjusting for sulfur atoms, and finally recalculating the coefficients to ensure an equal number of fluorine atoms. The summary highlights that the order of balancing elements can vary, and corrections can be made during the process until the correct balance is achieved.
🔥 Balancing Combustion Reactions: Propane, Oxygen, and Products
The fourth paragraph focuses on balancing a combustion reaction involving propane and oxygen gas to produce carbon dioxide and water. It outlines a strategy for balancing such reactions by first balancing carbon atoms, then hydrogen atoms, and finally oxygen atoms last. The summary provides a step-by-step guide to adjusting coefficients to balance the number of atoms for each element, emphasizing the avoidance of fractional coefficients by multiplying through by a denominator to achieve whole numbers.
🌐 Balancing Single and Double Replacement Reactions
The final paragraph covers the balancing of single and double replacement reactions. For the single replacement reaction between aluminum and copper chloride, the process involves balancing the chlorine atoms first and then adjusting coefficients for aluminum and copper to achieve equality. In the double replacement reaction to form sodium phosphate, the emphasis is on balancing polyatomic ions as single units, starting with the ion with the highest charge, which simplifies the process. The summary advises against separating polyatomic ions into individual elements and stresses the importance of viewing them as one unit.
Mindmap
Keywords
💡Chemical Equation
💡Balancing
💡Coefficient
💡Subscript
💡Magnesium Nitride
💡Ammonia
💡Combustion Reaction
💡Polyatomic Ion
💡Double Replacement Reaction
💡Least Common Multiple (LCM)
💡Fractional Coefficients
Highlights
Balancing a chemical equation involves ensuring an equal number of atoms on both sides of the equation.
Coefficients are used to adjust the number of atoms of an element in a chemical equation.
Subscripts should not be altered when balancing chemical equations.
The example of magnesium reacting with nitrogen gas to form magnesium nitride demonstrates the balancing process.
In the case of nitrogen gas and hydrogen gas forming ammonia, nitrogen atoms are balanced first, followed by hydrogen atoms.
For sulfur and fluorine gases forming sulfur hexafluoride, balancing fluorine atoms first simplifies the process.
In a combustion reaction, such as propane with oxygen to form CO2 and H2O, carbon atoms are balanced first, followed by hydrogen, and then oxygen last.
Fractional coefficients are not preferred; whole numbers should be used in balanced chemical equations.
The least common multiple (LCM) can be used to balance the number of atoms when balancing chemical equations.
In single replacement reactions, such as aluminum with copper chloride, balancing the number of atoms for each element is crucial.
For double replacement reactions involving polyatomic ions, it's recommended to balance ions rather than individual elements.
In double replacement reactions, balancing polyatomic ions or ions with the highest charge can simplify the balancing process.
The video provides practice problems and links for further practice on balancing chemical equations.
Balancing chemical equations is a fundamental skill in chemistry, essential for understanding chemical reactions.
The video explains the process of balancing equations step by step, making it accessible for learners at various levels.
Different types of chemical reactions, such as combustion and single replacement, require specific approaches to balancing.
The importance of not changing subscripts and using coefficients to balance equations is emphasized.
The video provides a clear explanation of how to balance complex chemical equations, including those with polyatomic ions.
The presenter offers strategies for balancing chemical equations efficiently, such as starting with the most complex ions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: