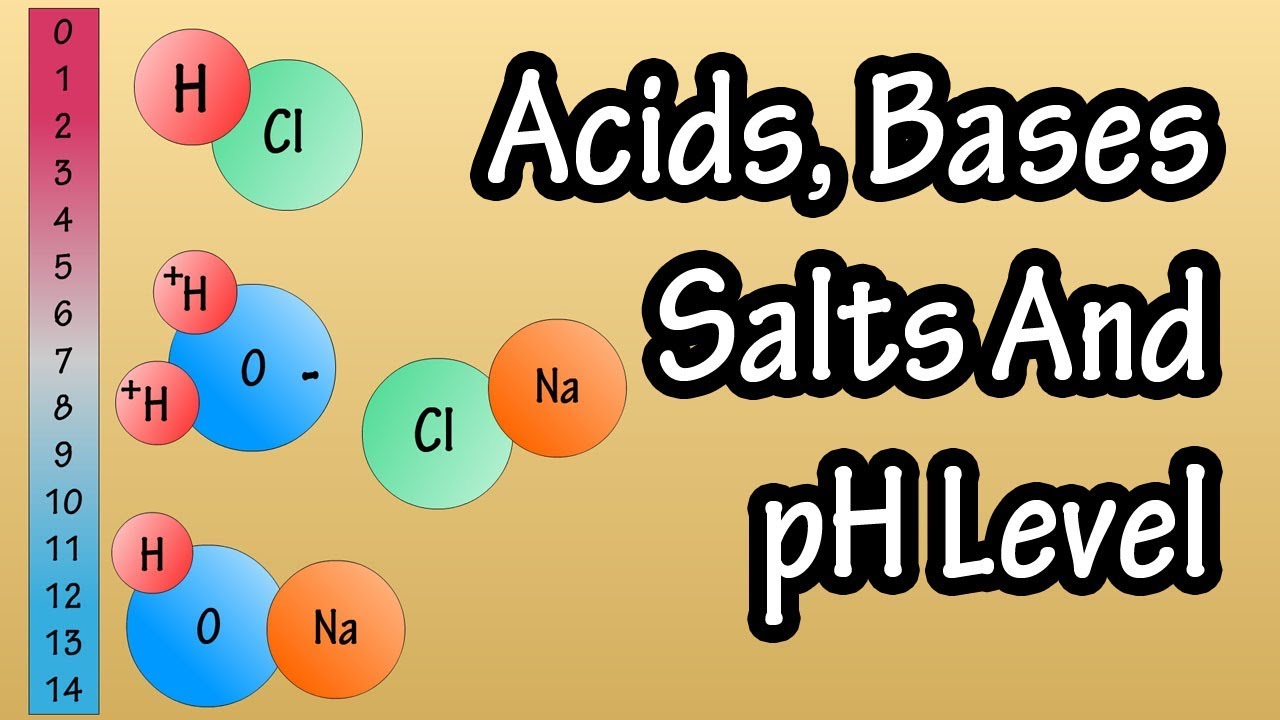
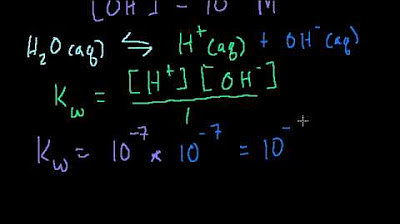PH Scale in Simple Terms
TLDRThe video script introduces the pH scale, a measure of hydrogen ion concentration in a substance, with 7 being neutral. Below 7 indicates acidity, while above 7 indicates bases. The script creatively uses mnemonic devices like 'lucky 7' and '7 heaven' to remember the neutral point. It also describes how acids and bases can be identified in everyday items, and their associated colors on the pH scale. The technical aspect of pH calculation is simplified, explaining that acids gain hydrogen ions and bases lose them. The video aims to educate viewers on the fundamental concept of pH in a relatable and engaging manner.
Takeaways
- 📌 pH stands for 'potential of hydrogen' and measures hydrogen ion concentration in a water-based substance.
- 📈 The pH scale ranges from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating alkalinity.
- 💡 Use mnemonic devices like 'lucky 7' or '7 is heaven' to remember that 7 is the neutral point on the pH scale.
- 🥛 Milk and apple juice are examples of slightly acidic substances, while lemon juice is more notably acidic.
- 🥚 Eggs, baking soda, ammonia, and bleach are examples of bases, which can taste bitter and have a slippery texture.
- 🎨 The pH scale is associated with colors: acids are yellow (turning to orange and red), while bases are green (turning to blue and purple).
- 🔬 Technically, pH is calculated as the negative logarithm of a solution's hydrogen ion concentration.
- 🔄 Acids gain hydrogen ions to form hydronium, while bases lose hydrogen ions to form hydroxide ions.
- 🔢 Each whole number on the pH scale represents a tenfold change in acidity or alkalinity; for example, a solution with a pH of 5 is 10 times more acidic than one with a pH of 6.
- 📚 For further understanding, follow educational content that regularly covers math and science topics, like moomoomath's daily video uploads.
Q & A
What does pH stand for?
-pH stands for the potential of hydrogen, which is a measure of the concentration of hydrogen ions in a water-based substance.
What is the pH scale range?
-The pH scale typically ranges from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating basicity.
Why is pH 7 considered neutral?
-pH 7 is considered neutral because it corresponds to the hydrogen ion concentration of pure water, which is neither acidic nor basic.
How can you remember which numbers on the pH scale indicate acidity or basicity?
-One can remember that 'lucky 7' or '7 is heaven' indicates neutrality. Any number below 7 is acidic, and any number above 7 is basic.
What are some examples of acidic substances mentioned in the script?
-Milk, apple juice, and lemon juice are mentioned as examples of acidic substances.
What are some examples of basic substances mentioned in the script?
-Eggs, baking soda, ammonia, and bleach are mentioned as examples of basic substances.
How do acids and bases affect the taste of substances?
-Acids can taste very sour, while bases may taste bitter and could also be slippery.
What color is associated with acids on the pH scale?
-Acids are associated with the colors yellow, orange, and red on the pH scale.
What color is associated with bases on the pH scale?
-Bases are associated with the colors green, blue, and almost purple on the pH scale.
How does the concentration of hydrogen ions differ between acidic and basic solutions?
-Acids gain hydrogen ions and create hydronium, while bases lose hydrogen ions and create hydroxide ions.
What does each whole number on the pH scale represent?
-Each whole number on the pH scale represents a tenfold change in the acidity or basicity of the solution.
How much more acidic is water with a pH of 5 compared to water with a pH of 6?
-Water with a pH of 5 is 10 times more acidic than water with a pH of 6.
Outlines
📚 Introduction to pH and its Scale
This paragraph introduces the concept of pH, explaining that it stands for the potential of hydrogen and represents the concentration of hydrogen ions in a water-based substance. The pH scale is briefly described, ranging from 0 to 14, with 7 being neutral. Acids are indicated by pH values less than 7, while bases are indicated by values greater than 7. A mnemonic device, 'lucky 7' or '7 heaven,' is suggested to remember that 7 is neutral on the pH scale. The paragraph also touches on the initial challenge of distinguishing between acidic and basic numbers on the scale.
🍎 Examples of Acidic and Basic Substances
This section delves into examples of substances that are acidic or basic. It mentions that milk and apple juice are slightly acidic, while lemon juice and stomach acid are also categorized as acids. On the other hand, eggs, baking soda, ammonia, and bleach are listed as examples of bases. The paragraph describes how some acids can taste very sour, whereas bases may taste bitter and have a slippery texture.
🎨 Visual Representation of pH Scale
The paragraph discusses the visual aspect of the pH scale, assigning colors to acids and bases. Acids are associated with the colors yellow, orange, and red, while bases are linked to green, blue, and almost purple. A mnemonic 'B for base and blue' is provided to help remember the color association with bases.
🧪 Technical Understanding of pH
This part of the script provides a technical explanation of pH, stating that it is calculated as the negative logarithm of a solution's hydrogen ion concentration. In simpler terms, it explains that acids gain hydrogen ions to form hydronium, while bases lose hydrogen ions to form hydroxide ions. The paragraph emphasizes that each whole number on the pH scale represents a tenfold change in acidity or basicity, using the example of water with a pH of 5 being ten times more acidic than water with a pH of 6.
📚 Further Learning and moomoomath's Offerings
The final paragraph encourages viewers to learn more about pH through a recommended playlist and highlights the availability of new math and science videos uploaded daily by moomoomath. It ends with a call to action for viewers to subscribe and share the content.
Mindmap
Keywords
💡pH scale
💡potential of hydrogen
💡hydrogen ions
💡acids
💡bases
💡neutral
💡hydronium
💡hydroxide
💡tenfold change
💡color association
💡negative log
Highlights
pH stands for the potential of hydrogen and is a measure of the concentration of hydrogen ions in a water-based substance.
The pH scale ranges from 0 to 14, with 7 being neutral, indicating the balance between acidic and basic substances.
Acidic substances have a pH less than 7, while basic substances have a pH greater than 7.
The concept of 'lucky 7' or '7 is heaven' on the pH scale is a mnemonic device to remember that 7 is neutral.
Milk and apple juice are examples of slightly acidic substances.
Lemon juice and stomach acid are examples of more pronounced acidic substances.
Eggs, baking soda, ammonia, and bleach are examples of basic substances.
Bases may have a bitter taste and can be slippery in texture.
The pH scale is color-coded, with acids ranging from yellow to red and bases from green to purple.
Acids are associated with the color yellow, progressing to orange and red.
Bases are associated with the color green, progressing to blue and almost purple.
pH is technically calculated as the negative log of a solution's hydrogen ion concentration.
Acids gain hydrogen ions and create hydronium, while bases lose hydrogen ions and create hydroxide.
Each number on the pH scale represents a tenfold change in acidity or basicness.
Water with a pH of 5 is 10 times more acidic than water with a pH of 6.
Moomoomath provides educational math and science videos daily, with a new video uploaded every day.
This playlist serves as a resource for those interested in learning more about pH and its applications.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: