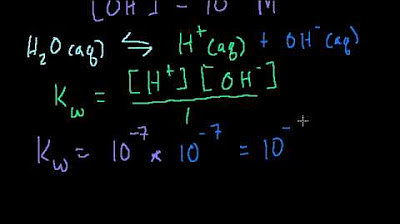Acids, Bases, and pH
TLDRThe video script delves into the concept of pH, explaining its significance in maintaining the stability of biological processes, particularly highlighting the role of pH in muscle function and protein activity. It introduces the molecular structure of water and its polar nature, leading to the formation of hydronium and hydroxide ions. The script then clarifies that pH measures the concentration of hydrogen ions, using the example of distilled water's pH being 7, acids having a pH lower than 7, and bases having a pH higher than 7. The impact of human activities, such as acid rain and ocean acidification due to increased carbon dioxide, is also discussed, emphasizing the environmental consequences and the importance of understanding pH in the context of ecological balance.
Takeaways
- 📚 pH is a measure of the hydrogen ion concentration in a solution, indicating its acidity or alkalinity.
- 💧 The pH scale ranges from 0 to 14, with 7 being neutral, values below 7 being acidic, and values above 7 being basic.
- 🥚 Myoglobin, a protein in muscles, is an example of how enzymes can be sensitive to pH levels, denaturing if pH deviates too far from its optimal level.
- 🌊 Water is a polar molecule, forming hydrogen bonds between its molecules, which contributes to its unique properties, including surface tension and cohesion.
- ⚡️ Hydronium ions (H3O+) are formed when a hydrogen atom is transferred from one water molecule to another, which is a rare occurrence in pure water.
- 🧪 The pH value is calculated as the negative logarithm of the hydrogen ion concentration, which simplifies the representation of these concentrations.
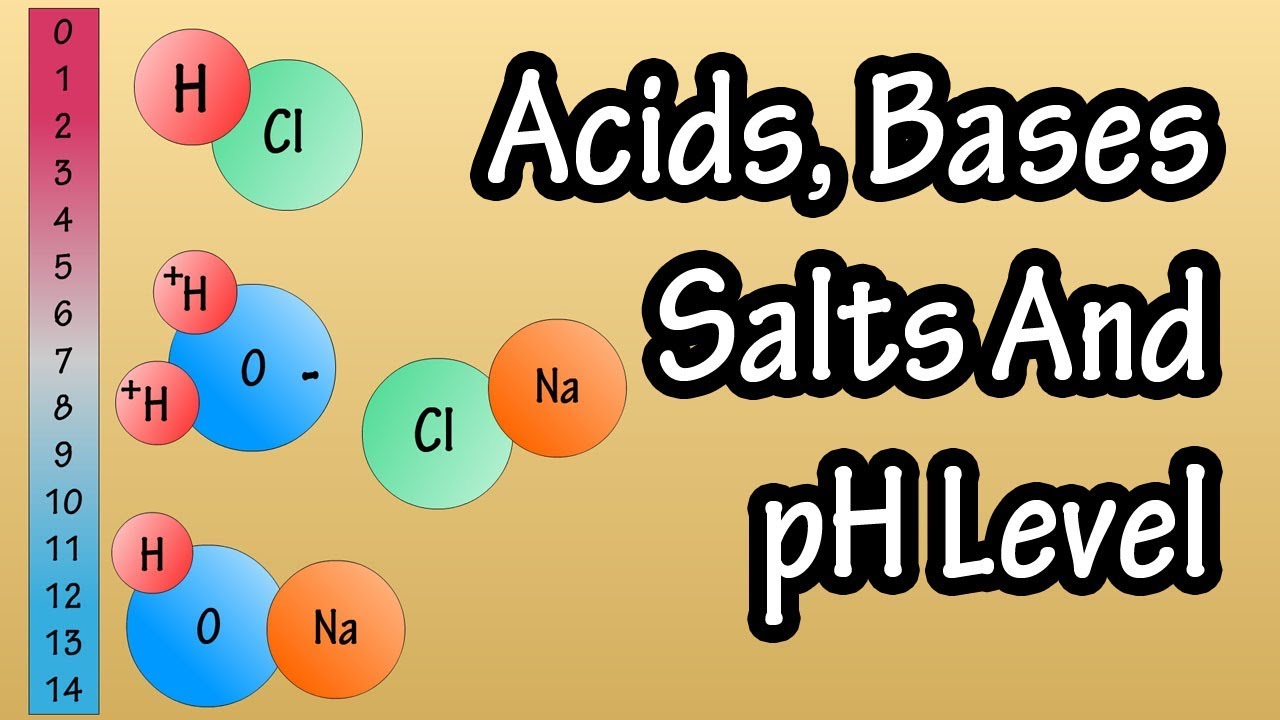
- 🍌 Acids, like hydrochloric acid, increase the concentration of hydrogen ions when dissolved in water, resulting in a lower pH value.
- 🧴 Bases, like sodium hydroxide, consume hydrogen ions when dissolved, leading to a higher pH value and a more basic solution.
- 🌧️ Acid rain and ocean acidification are real-world examples of how pH levels can change in the environment, impacting ecosystems and life forms.
- 🔍 Understanding pH is crucial for various applications, from maintaining biological processes to addressing environmental concerns.
Q & A
What does pH measure?
-pH measures the concentration of hydrogen ions (H+) or hydronium ions (H3O+) in a solution, indicating its acidity or alkalinity.
What is the pH value of distilled water?
-The pH value of distilled water is 7, which is considered neutral on the pH scale.
What happens to a protein like myoglobin if the pH level is too low or too high?
-If the pH level is too low or too high, the protein myoglobin will denature, and as a result, muscles will not function properly.
How does the polarity of water molecules contribute to the formation of hydrogen bonds?
-The polarity of water molecules, where oxygen has a partial negative charge and hydrogen has a partial positive charge, allows hydrogen atoms of one water molecule to be attracted to the oxygen of another, forming hydrogen bonds.
What is hydronium and how does it relate to pH?
-Hydronium is a positively charged ion (H3O+) that forms when a hydrogen atom detaches from one water molecule and attaches to another. The presence of hydronium ions in water affects the pH level, with higher concentrations leading to lower pH values (more acidic).
What is the relationship between the pH scale and the concentration of hydrogen ions?
-The pH scale is a logarithmic scale where a lower pH value indicates a higher concentration of hydrogen ions, making the solution more acidic, while a higher pH value indicates a lower concentration of hydrogen ions, making the solution more basic or alkaline.
How does the addition of hydrochloric acid to water affect the pH?
-Adding hydrochloric acid to water increases the concentration of hydrogen ions, resulting in a lower pH value, indicating that the solution becomes more acidic.
What occurs when sodium hydroxide is added to water, and how does it impact pH?
-Sodium hydroxide dissociates into sodium and hydroxide ions in water. The hydroxide ions react with hydrogen ions, reducing their concentration, which leads to a higher pH value, indicating that the solution becomes more basic.
How does acid rain affect the pH of the environment?
-Acid rain is caused by the release of certain acids into the atmosphere, which then fall to the ground and lower the pH of the environment, making it more acidic.
What is the impact of increasing carbon dioxide levels in the atmosphere on ocean pH?
-Increasing carbon dioxide levels in the atmosphere leads to more carbon dioxide combining with water to form carbonic acid, which increases the acidity of the oceans and results in a decrease in ocean pH.
How do changes in ocean pH affect marine life?
-Changes in ocean pH, particularly a decrease, can have significant effects on marine life, including the dissolution of coral reefs and the disruption of the delicate balance of marine ecosystems, potentially leading to mass extinctions.
Outlines
📚 Introduction to Acids, Bases, and pH
This paragraph introduces the topic of acids, bases, and pH, highlighting the common misconceptions about these concepts. Mr. Andersen, a biology teacher, emphasizes the importance of understanding pH in the context of life, using the example of myoglobin, a protein found in muscles. The video aims to explain what pH measures and why it's crucial for maintaining the proper functioning of proteins. The paragraph also delves into the chemistry of water, discussing its polar nature and how it leads to the formation of hydronium (H3O+) and hydroxide (OH-) ions. The concept of pH is defined as a measure of the power or concentration of hydrogen ions in a solution, with a pH of 7 representing a neutral solution. The explanation includes the role of water's polar covalent bonds and the significance of hydrogen bonding in the formation of hydronium ions, which are rare but impactful in water. The paragraph concludes with the equation for pH, which is the negative logarithm of the hydrogen ion concentration, and illustrates how this scale works with the example of distilled water having a pH of 7.
🍋 Understanding Acids and Bases
In this paragraph, the focus shifts to a deeper understanding of acids and bases, and how they affect pH levels. Mr. Andersen explains that acids, such as hydrochloric acid found in the stomach, increase the concentration of hydrogen ions (H+) when dissolved in water, leading to a lower pH value. The example of hydrochloric acid is used to demonstrate how the pH scale works, with varying concentrations of the acid resulting in different pH levels. The discussion then moves to bases, like sodium hydroxide, and their effect on pH. Bases combine with hydrogen ions, reducing their concentration and resulting in a higher pH value. The paragraph also addresses the common confusion between increasing hydrogen ion concentration (which lowers pH) and decreasing it (which raises pH). The importance of pH in environmental contexts, such as acid rain and ocean acidification, is highlighted, with the latter being linked to the increase in atmospheric carbon dioxide and its impact on marine life and the potential for mass extinctions. The paragraph concludes by reiterating the simplicity of the pH concept and its significance in various biological and environmental contexts.
Mindmap
Keywords
💡pH
💡Acids
💡Bases
💡Hydronium
💡Hydroxide Ion
💡Polar Molecule
💡Hydrogen Bond
💡Myoglobin
💡Acid Rain
💡Ocean Acidification
💡Log Scale
Highlights
pH is a measure of how acidic or basic a substance is, typically represented on a scale from 0 to 14.
Water has a pH of 7, which is considered neutral, neither acidic nor basic.
The pH scale is logarithmic, meaning each whole number change on the pH scale corresponds to a tenfold change in acidity or alkalinity.
A substance with a pH less than 7 is acidic, and the lower the pH, the more acidic it is.
A substance with a pH greater than 7 is basic, and the higher the pH, the more basic it is.
The myoglobin protein in muscles is most active at a pH of 6 and can denature if pH levels vary too much.
The polar nature of water molecules leads to the formation of hydrogen bonds, which contribute to water's cohesive properties.
Hydronium ions (H3O+) and hydroxide ions (OH-) are formed when water molecules gain or lose hydrogen atoms.
The pH of a solution is determined by the concentration of hydrogen ions (H+) or hydronium ions (H3O+) present.
The lower the concentration of hydrogen ions, the higher the pH and the more basic the solution.
Adding an acid like hydrochloric acid (HCl) to water increases the concentration of hydrogen ions, thus lowering the pH.
Adding a base like sodium hydroxide (NaOH) to water decreases the concentration of hydrogen ions, thus increasing the pH.
The concept of pH is crucial in understanding the effects of acid rain and ocean acidification.
Ocean acidification, caused by increased carbon dioxide in the atmosphere, can have significant impacts on marine life and ecosystems.
The pH scale and understanding of acids and bases is fundamental in the field of biology, particularly in relation to biochemical processes and environmental science.
The video explains the concept of pH in a simple and accessible manner, making complex scientific ideas understandable for a general audience.
The importance of maintaining a stable pH in biological systems is emphasized, highlighting the potential consequences of pH imbalances.
The video provides a clear explanation of the relationship between the concentration of hydrogen ions and the pH value of a solution.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: