What Are Acids & Bases? | Chemistry Basics
TLDRThis script explores the concept of acids, highlighting their presence in everyday life and their safety or danger based on hydrogen ion (H+) concentration. It introduces the pH scale, developed by Søren Sørensen in 1909, which measures acidity or alkalinity. The scale ranges from 0 to 14, with 7 being neutral. Acids are below 7, while bases, their chemical opposites, are above. Both can be harmful if strong. The script also touches on the importance of acids and bases in biochemistry, where they play crucial roles in biological processes and neutralization reactions, as well as the amphoteric nature of molecules like amino acids.
Takeaways
- 🍋 Acids are chemicals that can be found in various aspects of daily life, from food to cleaning products.
- ⚛️ The key component of acids is the hydrogen ion (H+), which is a hydrogen atom that has lost its electron.
- 💧 Acids produce H+ ions when dissolved in water, and the amount of H+ determines their safety for consumption.
- 📊 Søren Sørensen developed the pH scale in 1909 to quantify acidity, with numbers ranging from 0 to 14.
- 📉 A lower pH value indicates a higher concentration of H+ ions, making the substance more acidic.
- ⚖️ The pH scale is logarithmic, meaning each unit difference represents a tenfold change in H+ concentration.
- 🌡 Substances with pH values above 7 are called bases and are the chemical opposites of acids.
- 💧 A pH of 7 represents a neutral solution, like pure water.
- 🧼 Bases, like ammonia and baking soda, can also be found in everyday life and can be harmful if strong.
- 🔁 Acids produce H+ ions in water, while bases produce OH- ions, and they can neutralize each other in reactions.
- 🧬 Acids and bases play crucial roles in biochemistry, with biological processes often being pH-sensitive.
Q & A
What is the main characteristic of acids that differentiates them from other chemicals?
-Acids are characterized by their ability to produce hydrogen ions (H+) when dissolved in water.
Who developed the pH scale and when was it developed?
-Søren Sørensen, a Danish chemist, developed the pH scale in 1909.
What is the pH scale's range and what does it represent?
-The pH scale ranges from 0 to 14, representing the concentration of hydrogen ions in a solution, with 0 being the most acidic and 14 being the most basic.
How does the pH scale work in relation to acids and bases?
-Acidic substances have a pH below 7, while basic substances have a pH above 7. A pH of 7 represents a neutral substance, like pure water.
What does it mean for the pH scale to be logarithmic?
-A logarithmic pH scale means that each unit difference represents a tenfold change in hydrogen ion concentration, for example, a solution with a pH of 3 is ten times more acidic than one with a pH of 4.
What is the chemical difference between acids and bases?
-Acids produce hydrogen ions (H+), while bases produce hydroxide ions (OH-) in water.
What happens during a neutralization reaction between acids and bases?
-In a neutralization reaction, hydrogen ions (H+) from the acid react with hydroxide ions (OH-) from the base to form water.
Why is controlling acidity in the body important for biological processes?
-Many biological processes are sensitive to pH, so maintaining the correct acidity is crucial for the proper functioning of these processes.
What is an example of an amphoteric molecule and why is this property significant?
-Amino acids are an example of amphoteric molecules because they can act as both acids and bases, having a carboxylic acid group to produce H+ and an amino group to accept H+. This property allows them to participate in various biochemical reactions.
How do the properties of acids and bases relate to their safety for consumption?
-The safety of consuming acids or bases depends on their strength and concentration. Strong acids and bases can be corrosive and dangerous, while weaker ones may be safe to eat, such as certain acidic fruits.
What are some common examples of bases found in everyday life?
-Examples of bases in everyday life include cleaning products like ammonia, baking soda, and some simple medicines like milk of magnesia.
Outlines
🍏 Acids in Daily Life and the pH Scale
This paragraph introduces the concept of acids, which are chemicals found in various aspects of daily life, from edible fruits to corrosive cleaning agents. It explains that the safety of consuming an acid depends on the amount of hydrogen ions (H+) it can produce when dissolved in water. Søren Sørensen's development of the pH scale in 1909 is highlighted as a method to quantify acidity by converting hydrogen ion concentration into a numerical scale from 0 to 14. Acidic substances have a pH below 7, with lower numbers indicating higher H+ concentrations. The scale is logarithmic, meaning each unit difference represents a tenfold change in H+ concentration. The paragraph also contrasts acids with bases, which are their chemical opposites, and explains that bases can be just as dangerous as acids. Bases are characterized by their bitter taste and slippery feel and produce OH- ions in water, which can neutralize acids in a neutralization reaction to form water.
Mindmap
Keywords
💡Acids
💡Hydrogen Ion (H+)
💡pH Scale
💡Logarithmic Scale
💡Bases
💡Neutral pH
💡Neutralization Reaction
💡Biochemistry
💡Amino Acids
💡Amphiprotic
💡Hydroxide Ion (OH-)
Highlights
Acids are chemicals found in many aspects of daily life, with some safe to eat and others dangerous.
Acids are characterized by the presence of hydrogen ions (H+), which they release when dissolved in water.
The pH scale, developed by Søren Sørensen in 1909, quantifies acidity by measuring hydrogen ion concentration in a solution.
The pH scale ranges from 0 to 14, with acidic substances having pH values below 7.
A one-unit difference on the pH scale represents a tenfold difference in hydrogen ion concentration.
Substances with pH values above 7 are called bases and are the chemical opposites of acids.
A pH of 7 represents a neutral substance, like pure water.
Bases, like acids, can be found in everyday chemicals and can be strong or weak.
Strong bases can be as dangerous and corrosive as strong acids.
Chemically, acids produce hydrogen ions (H+), while bases accept them.
In water, acids produce H+ ions and bases produce OH- ions.
A neutralization reaction occurs when hydrogen ions react with hydroxide ions to form water.
Acids and bases play a crucial role in biochemistry, with many biological processes being sensitive to pH levels.
Controlling acidity in the body is essential for various biological functions.
Amino acids are amphoteric molecules, capable of acting as both acids and bases.
Amino acids have a carboxylic acid group that produces hydrogen ions and an amino group that accepts them.
Acids and bases are involved in the transfer of hydrogen ions in various chemical and biological processes.
The video provides an introduction to the basics of chemistry, focusing on the properties and reactions of acids and bases.
Transcripts
Browse More Related Video

PH Scale in Simple Terms
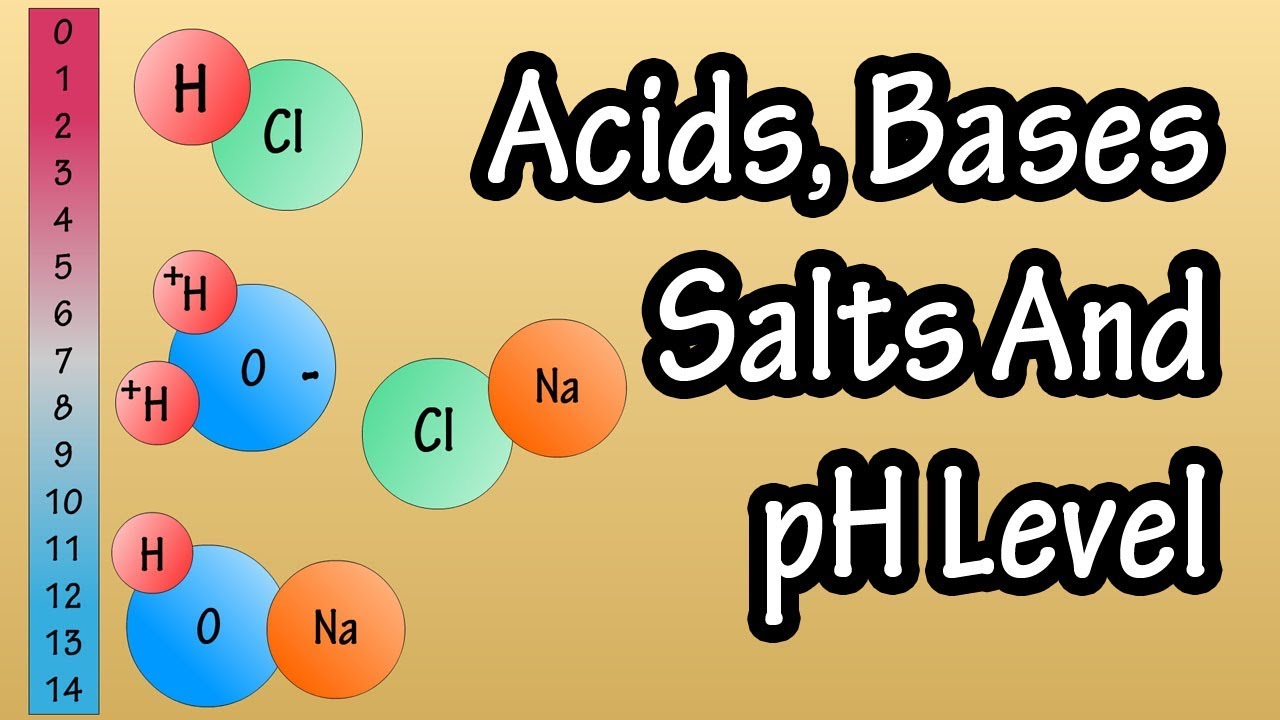
Acids And Bases Salts And pH Level - What Are Acids Bases And Salts - What Is The pH Scale Explained

ASL Acids and Bases for Kids
pH and pOH: Crash Course Chemistry #30

What is pH ; How to Calculate pH (3 examples) - Chemistry

Acids and Bases for Kids | Learn the difference between an acid and a base
5.0 / 5 (0 votes)
Thanks for rating: