Introduction to pH, pOH, and pKw
TLDRThe video script discusses the autoionization of water, a spontaneous process where water molecules react with each other, forming hydronium and hydroxide ions. It explains the equilibrium constant (Kw) and how it is used to derive the pH and pOH values of water at room temperature, both of which are 7. The script also introduces the concept of acids and bases, noting that acids increase the hydrogen ion concentration, thereby lowering the pH, while bases increase the hydroxide ion concentration.
Takeaways
- 🌊 The fundamental concept in chemistry is the random interaction between molecules, which can lead to various chemical reactions, including in pure water.
- 🔄 In water, there is a natural process of autoionization where water molecules can ionize into hydroxide (OH-) and hydronium (H3O+) ions through random collisions.
- 🔋 The oxygen in water molecules has a partial negative charge due to its higher electronegativity, while the hydrogen atoms have a partial positive charge, facilitating the formation of hydrogen bonds.
- 📈 The equilibrium constant for the autoionization of water (Kw) at 25°C is 1 x 10^-14, indicating the relative concentrations of H3O+ and OH- ions in pure water.
- 📊 At room temperature, the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in pure water is 1 x 10^-7 moles per liter.
- 📈 The pH scale is derived from the negative logarithm (base 10) of the hydrogen ion concentration, where a pH of 7 corresponds to a neutral solution.
- 📈 The pOH scale is the negative logarithm (base 10) of the hydroxide ion concentration, which is also 7 at room temperature for pure water.
- 🔄 The autoionization of water is a dynamic equilibrium, meaning that the forward and reverse reactions occur simultaneously, maintaining a constant ratio of ion concentrations.
- 🧪 The addition of an acid to water increases the concentration of hydrogen ions, resulting in a decrease in pH, while the addition of a base increases the concentration of hydroxide ions, leading to an increase in pH.
- 🔢 The pKw value of 14 at 25°C represents the equilibrium constant for the autoionization of water, which is the negative logarithm of the product of the concentrations of H3O+ and OH- ions.
- 📚 Understanding the autoionization of water and the resulting pH and pOH values is crucial for studying the behavior of acids and bases in aqueous solutions.
Q & A
What is the main theme of the chemistry concept discussed in the transcript?
-The main theme is the autoionization of water, which is a spontaneous process where water molecules can ionize into hydronium and hydroxide ions due to random molecular collisions.
How does the partial positive charge on the hydrogen side of water molecules contribute to the formation of hydrogen bonds?
-The partial positive charge on the hydrogen side of water molecules allows them to form hydrogen bonds with the partial negative charge on the oxygen side of another water molecule, which helps keep water in its liquid or solid states.
What is the equilibrium reaction representing the autoionization of water?
-The equilibrium reaction is H2O (aq) ⇌ H3O+ (aq) + OH- (aq), showing the balance between water molecules and the ions formed through autoionization.
What is the significance of the equilibrium constant (Kw) for the autoionization of water?
-The equilibrium constant (Kw) represents the ratio of the concentrations of the products (hydronium and hydroxide ions) to the concentration of the reactants (water molecules) at equilibrium. It is a fixed value that helps determine the extent of the autoionization process in water.
What is the value of the equilibrium constant (Kw) for water at 25 degrees Celsius?
-The value of Kw for water at 25 degrees Celsius is 10^-14 moles^2/liter^2.
How is the pH scale related to the hydrogen ion concentration in a solution?
-The pH scale is derived from the negative logarithm (base 10) of the hydrogen ion concentration. A lower pH indicates a higher concentration of hydrogen ions, making the solution more acidic.
What is the pH and pOH of pure water at room temperature?
-The pH and pOH of pure water at room temperature are both equal to 7, indicating a neutral solution.
What happens to the pH of a solution when an acid is added to it?
-When an acid is added to a solution, it increases the hydrogen ion concentration, resulting in a lower pH value, indicating the solution has become more acidic.
How does the concentration of hydronium ions change when the pH of a solution decreases?
-As the pH of a solution decreases, the concentration of hydronium ions increases, because pH is the negative logarithm of the hydronium ion concentration.
What is the relationship between pKw and the autoionization constant (Kw) of water?
-The pKw is the negative logarithm (base 10) of the autoionization constant (Kw) of water. At room temperature, pKw is equal to 14, which corresponds to the Kw value of 10^-14.
How does the addition of a base to water affect the pH and pOH values?
-Adding a base to water increases the concentration of hydroxide ions, which leads to a decrease in the hydrogen ion concentration and an increase in pH (making the solution more basic). Concurrently, the pOH value decreases because pOH is the negative logarithm of the hydroxide ion concentration.
Outlines
🔬 Autoionization of Water
This paragraph delves into the concept of autoionization in chemistry, focusing on water molecules. It explains how random collisions between water molecules can lead to the formation of hydronium (H3O+) and hydroxide (OH-) ions. The process is described as a spontaneous and equilibrium reaction where water molecules can ionize and then recombine. The paragraph emphasizes that this reaction is probabilistic and occurs with a very small number of molecules, and it introduces the equilibrium equation for the reaction. It also touches on the importance of understanding this process in relation to the broader context of chemistry.
🧪 Equilibrium in Aqueous Solutions
This section builds upon the autoionization of water, discussing the equilibrium between water molecules, hydronium ions, and hydroxide ions in an aqueous solution. It clarifies that 'aqueous solution' refers to a solution where the solute is dissolved in water. The paragraph explains that the hydrogen ions (protons) do not exist alone in water but instead form hydronium ions by associating with water molecules. It also introduces the concept of the equilibrium constant (Kw) for the autoionization of water and provides its value at room temperature. The discussion sets the stage for understanding the behavior of acids and bases in water.
📈 Calculating the pKw and pH
This paragraph introduces the pKw and pH concepts, which are crucial in understanding the acidity or basicity of solutions. It explains that pKw is the negative logarithm (base 10) of the equilibrium constant (Kw) for the autoionization of water, which equals 14 at room temperature. The paragraph then connects this to the pH scale, which is the negative logarithm of the hydrogen ion concentration. It establishes that at room temperature, the pH and pOH of pure water are both 7, indicating neutrality. The explanation includes a method for calculating the pH when the hydrogen ion concentration changes, demonstrating how adding an acid to water lowers the pH value.
🌡️ Impact of Acids on pH Levels
The final paragraph discusses the effect of acids on the pH of water. It defines an acid as a substance that increases the hydrogen ion concentration in a solution. By increasing the hydrogen ion concentration, an acid will lower the pH of the solution. The paragraph provides a hypothetical example of how adding hydrogen to water decreases the pH value from 7 (neutral) to 3 (acidic). It also briefly mentions the concept of bases, which will be covered in subsequent videos, and how they increase the hydroxide ion concentration, implying an increase in pH. The paragraph concludes with a clear takeaway: the importance of hydrogen ion concentration in determining the acidity of a solution.
Mindmap
Keywords
💡Chemistry
💡Molecules
💡Autoionization
💡Hydrogen Bond
💡Equilibrium
💡pH
💡pOH
💡Hydronium Ion
💡Hydroxide Ion
💡Equilibrium Constant (Kw)
💡Avogadro's Number
Highlights
The consistent theme in chemistry is about random interactions between molecules.
Random molecular interactions can lead to various outcomes, including changes in molecular structure.
Even pure water, considered a stable substance, undergoes random molecular interactions.
Molecular interactions can result in the formation of equilibrium equations, representing the ratios of molecules.
Water molecules have a partial positive charge on the hydrogen side and a partial negative charge on the oxygen side due to electronegativity.
Hydrogen bonding between water molecules is what keeps them together in liquid or solid states.
Autoionization is the spontaneous ionization of water molecules due to random molecular interactions.
In autoionization, one water molecule loses a proton and becomes hydroxide (OH-), while another gains a proton to become hydronium (H3O+).
The equilibrium constant for the autoionization of water (Kw) at 25°C is 1 x 10^-14.
The concentration of hydrogen ions (H+) and hydroxide ions (OH-) in pure water at 25°C is 1 x 10^-7 moles per liter.
The pH and pOH of water at room temperature are both equal to 7, indicating a neutral solution.
The pKw, which is the negative log of the equilibrium constant for water autoionization, is 14 at 25°C.
Acids are substances that increase the hydrogen ion concentration in a solution, thus lowering the pH.
Bases are substances that increase the hydroxide ion concentration, which is the opposite of acids.
The concept of pH is a measure of the hydrogen ion concentration and is used to determine the acidity or basicity of a solution.
The autoionization of water is a fundamental concept leading to the understanding of acids and bases.
The equilibrium between hydrogen and hydroxide ions in water is dynamic, with the ions constantly forming and rejoining to form water molecules.
Transcripts
Browse More Related Video

Acid Base Introduction
pH and pOH: Crash Course Chemistry #30
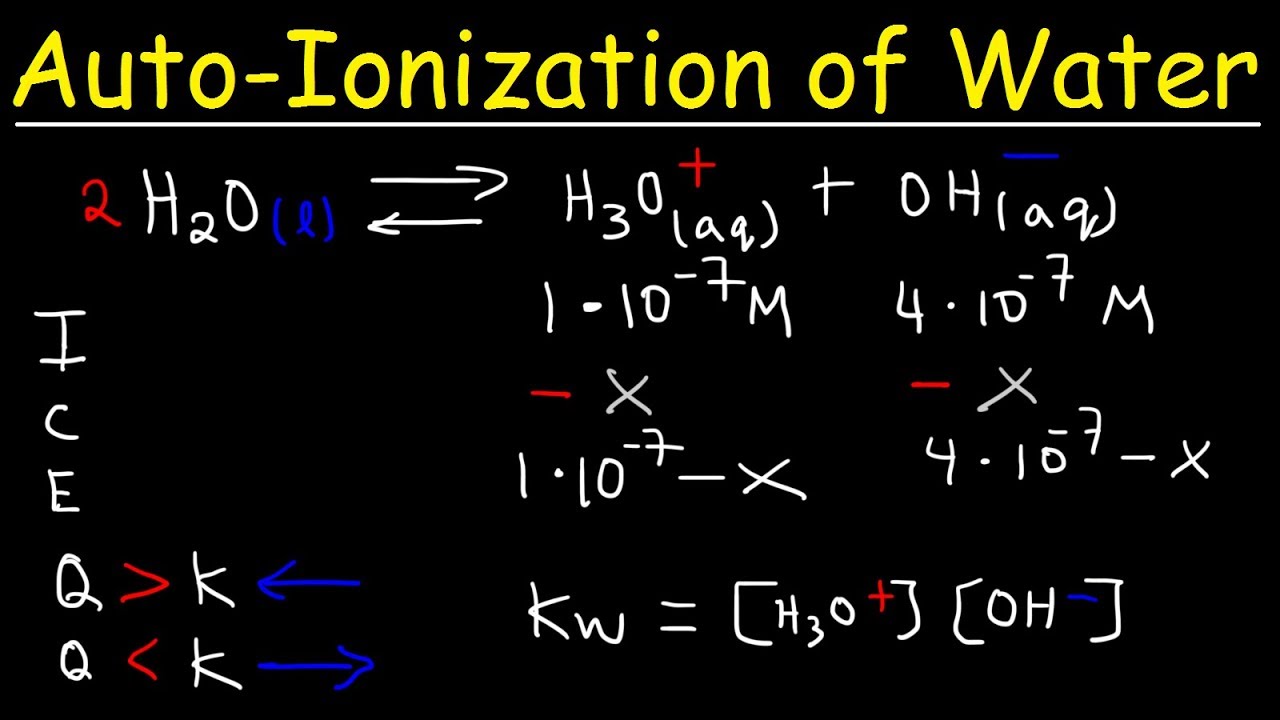
AutoIonization of Water, Ion Product Constant - Kw, Calculating H3O+, OH-, and pH Using Ice Tables

pH, pOH of strong acids and bases | Chemistry | Khan Academy

Acids and Bases, pH and pOH
Acids And Bases Salts And pH Level - What Are Acids Bases And Salts - What Is The pH Scale Explained
5.0 / 5 (0 votes)
Thanks for rating: