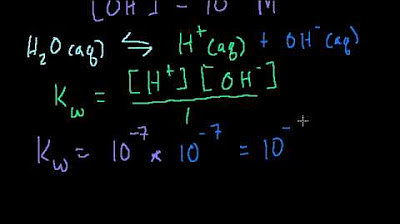What is pH ; How to Calculate pH (3 examples) - Chemistry
TLDRThis script delves into the concept of pH, a crucial measure for the strength of acids and bases. It introduces the Arrhenius and Brønsted-Lowry definitions, explaining how they relate to the generation and acceptance of H+ ions. Søren Sørensen's pH scale is detailed, illustrating how it logarithmically quantifies H+ ion concentration. The script clarifies that water, with a pH of 7, is neutral due to its minimal auto-ionization. It also explains how pH values below or above 7 indicate acidity or basicity, respectively. Practical examples, such as lemon juice and washing detergent, demonstrate how to calculate H+ ion concentration and interpret pH values, providing a tangible understanding of these concepts.
Takeaways
- 🧪 pH is a scale used to measure the strength of acids and bases.
- 📝 pH paper is a tool for quickly determining if a solution is acidic or basic through color change.
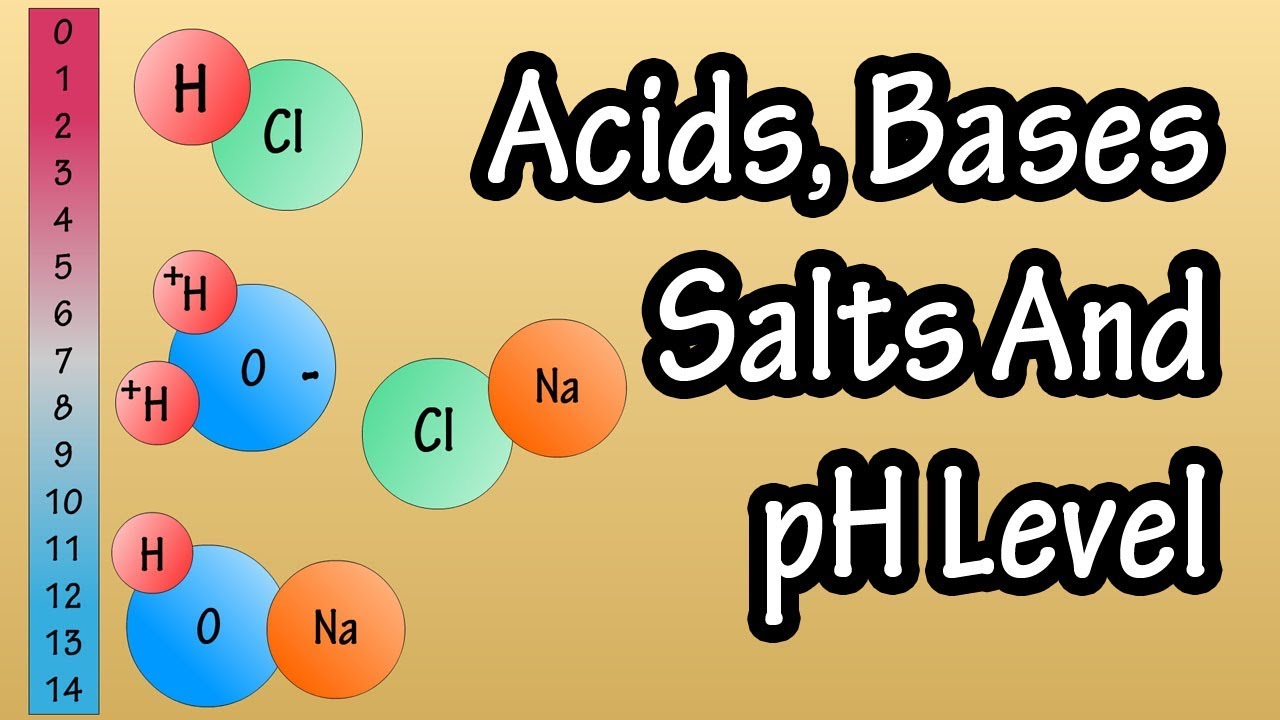
- 🌟 Arrhenius acids generate H+ ions when dissolved in water, while bases generate OH- ions.
- 🔬 The Brønsted-Lowry definition of acids and bases is based on the transfer of a proton (H+).
- 🌍 The Brønsted-Lowry definition is more general and does not require substances to be dissolved in water.
- 📊 The pH scale is a logarithmic scale that simplifies the expression of very small H+ ion concentrations.
- ⚗️ The equation for pH is pH = -log [H+], where [H+] is the concentration of hydrogen ions in moles per liter.
- 💧 Pure water slightly donates and accepts protons, undergoing auto-ionization, and is considered neutral with a pH of 7.
- 🔢 A pH of 7 indicates an equal concentration of H+ and OH- ions, while a pH less than 7 indicates an acidic solution and a pH greater than 7 indicates a basic solution.
- 🔄 The concentration of H+ and OH- ions in a solution are interdependent, with the product of their concentrations being a constant (1 x 10^-14 for aqueous solutions).
- 🍋 Lemon juice, with a pH of 2, has a higher concentration of H+ ions than pure water, making it acidic.
- 🧴 Washing detergent, with a pH of 8.5, is basic and has a lower concentration of H+ ions compared to acidic substances like lemon juice.
- 🥤 Soda with a pH of 2.5 is acidic, indicating a higher concentration of H+ ions than in neutral water.
Q & A
What is pH and why is it important?
-pH is a scale used to measure the acidity or basicity of a solution. It's important because it helps us determine the strength of acids and bases and understand their chemical behavior in various applications.
What is an Arrhenius acid and how does it relate to water?
-An Arrhenius acid is a substance that generates H+ ions when dissolved in water. It relates to water because the Arrhenius definition specifically deals with aqueous solutions of substances.
How does the Brønsted-Lowry definition of acids and bases differ from the Arrhenius definition?
-The Brønsted-Lowry definition is based on the transfer of a proton (H+ ion) from one substance to another, with a Brønsted-Lowry acid releasing a proton and a base accepting it. This differs from the Arrhenius definition, which specifically requires the substances to be dissolved in water.
Who proposed the pH scale and what is its purpose?
-The pH scale was suggested by Søren Sørensen. Its purpose is to provide a logarithmic scale that makes it easier to express the very small amounts of H+ ions produced by acids and bases.
What is the equation used to calculate pH and what does it represent?
-The equation used to calculate pH is pH = -log [H+]. It represents the negative logarithm of the hydrogen ion concentration in moles per liter.
Is water considered an acid, a base, or neither? Why?
-Water is considered neither an acid nor a base. It undergoes a very slight auto-ionization, producing a small amount of H+ and OH- ions, but the concentration of these ions is balanced, making water neutral.
What is the pH of pure water and what does it indicate?
-The pH of pure water is 7, which indicates that it is neutral with an equal concentration of H+ and OH- ions.
How does the pH scale work in terms of acidity and basicity?
-On the pH scale, a pH less than 7 indicates an acidic solution with more H+ ions than OH- ions, while a pH greater than 7 indicates a basic solution with more OH- ions than H+ ions.
What is the relationship between the concentrations of H+ and OH- ions in aqueous solutions?
-In aqueous solutions, the product of the concentrations of H+ and OH- ions is a constant, 1 x 10^-14. This means that the more H+ ions there are, the fewer OH- ions, and vice versa.
How can you calculate the concentration of H+ ions from the pH value?
-You can calculate the concentration of H+ ions from the pH value using the equation: [H+] = 10^(-pH). For example, if pH = 2, then [H+] = 10^(-2) = 0.01 moles per liter.
What is the significance of the negative sign in the pH equation?
-The negative sign in the pH equation ensures that as the concentration of H+ increases (making the solution more acidic), the pH value decreases, which is a more intuitive way to express acidity.
Can you provide an example of a real-world acidic substance and its pH?
-Lemon juice is an example of a real-world acidic substance with a pH of 2, indicating a relatively high concentration of H+ ions.
How does the pH of washing detergent compare to that of lemon juice?
-Washing detergent has a pH of around 8.5, which is basic, compared to lemon juice with a pH of 2, which is acidic. The detergent has a much lower concentration of H+ ions than lemon juice.
What can you infer about a soda with a pH of 2.5?
-A soda with a pH of 2.5 is quite acidic, as it has a higher concentration of H+ ions compared to neutral water with a pH of 7.
What are some average pH values of common substances and why might some be surprising?
-The average pH values of common substances can range from very acidic (like lemon juice with pH 2) to very basic (like household ammonia with pH around 11). Some might be surprising because they don't align with our everyday perceptions of acidity or basicity.
Outlines
🧪 Understanding Acids and Bases with pH
This paragraph introduces the concept of pH as a measure for the strength of acids and bases. It explains the use of pH paper in labs for quick identification of acidic or basic solutions. The definitions of Arrhenius acids and bases are provided, which are substances that generate H+ and OH- ions respectively when dissolved in water. The Brønsted-Lowry definition is also discussed, which is based on the transfer of a proton. The paragraph then focuses on the pH scale, introduced by Søren Sørensen, and explains its logarithmic nature, with the equation pH = -log [H+]. The auto-ionization of water is described, which leads to a neutral pH of 7, and the relationship between pH values and the concentrations of H+ and OH- ions is established, with the product of these ions being a constant in aqueous solutions.
🍋 Real-World Applications of pH Measurement
This paragraph delves into practical examples of how pH is used to determine the acidity or basicity of substances. It explains how to calculate the concentration of H+ ions using the pH formula and provides examples with substances like lemon juice (pH 2), washing detergent (pH 8.5), and a hypothetical soda (pH 2.5). The paragraph illustrates the concept that a lower pH indicates a higher concentration of H+ ions, making the solution more acidic, while a higher pH signifies a lower concentration of H+ ions, indicating a more basic solution. It also emphasizes the exponential relationship between pH values and H+ ion concentrations, highlighting the significant differences in acidity or basicity even with small changes in pH.
Mindmap
Keywords
💡pH
💡Arrhenius acid
💡Bases
💡Brønsted-Lowry acid
💡Brønsted-Lowry base
💡Auto-ionization
💡Søren Sørensen
💡Logarithmic scale
💡Concentration of H+ ions
💡Neutral solution
💡pH of common substances
Highlights
pH is a measure of the strengths of acids and bases.
pH paper is used to determine if a solution is acidic or basic.
Arrhenius acid generates H+ ions when dissolved in water.
Arrhenius base generates OH- ions when dissolved in water.
Brønsted-Lowry definition is based on the transfer of a proton.
A Brønsted-Lowry acid releases a proton.
A Brønsted-Lowry base accepts a proton.
pH scale was suggested by Søren Sørensen and is a logarithmic scale.
pH scale equation is pH = -log [H+].
Water slightly donates and accepts protons through auto-ionization.
Water is considered neutral with a pH of 7.
pH less than 7 indicates an acid, greater than 7 indicates a base.
Concentration of H+ and OH- ions are interdependent.
Product of H+ and OH- ions in aqueous solutions is constant.
Each pH difference represents a 10-fold difference in H+ ion concentration.
Lemon juice has a pH of 2 and a high concentration of H+ ions.
Washing detergent has a pH of 8.5 indicating it is a base.
Soda with a pH of 2.5 has a high concentration of H+ ions.
Average pH values of common substances are provided.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: