M - Steam Table Basics
TLDRThe video script provides a basic orientation on steam tables, focusing on enthalpy values in BTUs per pound. It explains the heat flow equation for steam, using enthalpy values to calculate energy requirements for processes like heating water to steam and condensing steam back to water. Practical examples illustrate how to apply these concepts in real-world scenarios.
Takeaways
- 📚 The session provides an introduction to steam tables, focusing on enthalpy values for energy calculations.
- 🔍 Enthalpy is a key parameter in steam tables, measured in BTUs per pound, and is denoted by H for both fluid and gaseous states.
- 🌡 The script explains the difference between enthalpy in the fluid state (Hf) and gaseous state (Hg), and the phase change enthalpy (Hfg).
- 🔄 Hfg represents the energy required to convert water into steam or vice versa, indicating the energy input for steam generation and energy released during condensation.
- 📈 The script demonstrates that the enthalpy of water increases linearly with temperature, with 1 BTU per pound per degree Fahrenheit.
- 💧 At the boiling point of water (212 degrees Fahrenheit), the enthalpy is 180 BTUs per pound, requiring an additional 970 BTUs per pound to convert to steam.
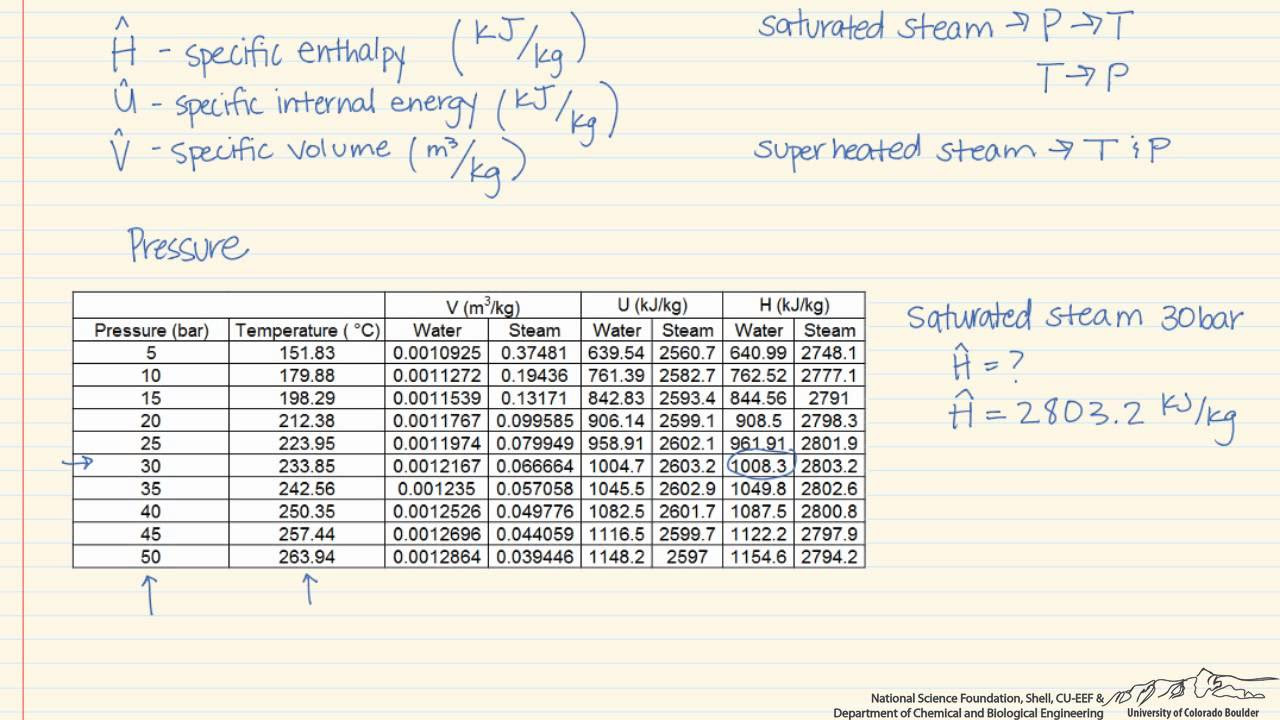
- 🌞 Saturated steam tables can be used to find enthalpy values when temperature or pressure is known, as saturated steam represents 100% humidity.
- 📊 The steam tables are organized by temperature and pressure, allowing for quick lookup of enthalpy values for given conditions.
- ⚙ The heat flow equation for steam (Q = m * ΔH) is discussed, showing how to calculate the energy required for phase changes using enthalpy values from the tables.
- 🔧 Two practical examples are provided: calculating the size of a boiler needed to heat water to steam and determining the heat released when steam condenses back to water.
- 🌐 The principles and calculations are applicable in both Imperial and SI units, with the main difference being the units used for measurements.
Q & A
What is the primary focus of the energy-related information in steam tables?
-The primary focus of the energy-related information in steam tables is the enthalpy, which is always measured in BTUs per pound.
What does the term 'H' in steam tables represent?
-In steam tables, 'H' represents enthalpy, which can be in the fluid state (Hf) or the gaseous state (Hg), and the phase change enthalpy (Hfg) represents the energy required for the phase transition from water to steam or vice versa.
How is the enthalpy of water linearly related to its temperature?
-The enthalpy of water is linearly related to its temperature because it increases by 1 BTU per pound for each degree Fahrenheit increase in temperature.
What is the enthalpy of water at its freezing point in the provided script?
-The enthalpy of water at its freezing point (32 degrees Fahrenheit) is essentially zero, as indicated in the script.
What is the significance of Hfg in the context of steam tables?
-Hfg signifies the enthalpy of the phase change, indicating the amount of energy required to convert water into steam at the same temperature, measured in BTUs per pound.
How can you determine the enthalpy of saturated steam given its temperature or pressure?
-You can determine the enthalpy of saturated steam by knowing either its temperature or pressure, as the steam tables are organized to provide these values directly.
What is the purpose of the heat flow equation for steam?
-The heat flow equation for steam incorporates enthalpy values to calculate the amount of heat (Q) required to change the state of water or steam, using the formula Q = m * ΔH.
In the script, what is the mass flow rate of water being heated to saturated steam in the boiler example?
-In the boiler example provided, the mass flow rate of water being heated to saturated steam is 100,000 pounds per hour.
What is the final enthalpy state when 60-degree water is heated to 400-degree saturated steam according to the script?
-The final enthalpy state when 60-degree water is heated to 400-degree saturated steam is 1201 BTUs per pound, as per the script.
How is the pressure in steam tables denoted, and what does it represent?
-The pressure in steam tables is denoted as 'psia', which stands for pounds per square inch absolute. It represents the gauge pressure plus 14.6, accounting for atmospheric pressure.
What is the significance of the condensate's pressure in the second example of the script?
-The significance of the condensate's pressure (20.7 psia) in the second example is to determine the enthalpy of the water state after the steam has been converted back to water, which is necessary to calculate the heat liberated by the process.
Outlines
🔍 Introduction to Steam Tables and Enthalpy Basics
This paragraph introduces the concept of steam tables, focusing on the importance of enthalpy in energy calculations. It explains that enthalpy is measured in BTUs per pound and is denoted differently for fluid and gaseous states (Hf and Hg respectively). The paragraph also discusses the significance of Hfg, which represents the enthalpy change during the phase transition from water to steam and vice versa. The speaker provides a practical example of how to read enthalpy values from the steam tables, including the linear relationship between temperature and enthalpy in the fluid state, and how to calculate the energy required to convert water into steam at a given temperature. The concept of saturated steam is introduced, explaining its relation to temperature and pressure, and how these properties can be used to find enthalpy values in the steam tables.
📚 Application of Steam Tables in Heat Flow Equations
The second paragraph delves into the application of steam tables in calculating heat flow for steam systems. It outlines a boiler example where water at 60 degrees Fahrenheit is heated to become 400-degree saturated steam, and the task is to determine the required heat input (Q). The speaker guides through the process of finding the initial and final enthalpy values from the steam tables, and then uses the heat flow equation (Q = m * ΔH) to calculate the necessary energy. The example is followed by another scenario where the steam produced is later condensed back into water at a specific pressure, and the paragraph explains how to calculate the heat released during this process using the enthalpy values from the pressure tables. The speaker concludes by summarizing the key points covered in the session, including the use of steam tables, heat flow equations, and enthalpy applications in relation to temperature and pressure, and hints at the similarity of processes in SI units.
Mindmap
Keywords
💡Steam Tables
💡Enthalpy
💡Saturated Steam
💡Heat Flow Equation
💡Boiler
💡Mass Flow Rate
💡Condensate
💡Pressure (psia)
💡Hvap (Enthalpy of Vaporization)
💡Temperature
💡HVAC
Highlights
Introduction to steam tables and their basic orientation for energy purposes.
Explanation of the heat flow equation for steam and its importance in energy calculations.
Detailed discussion on enthalpy, its units, and its significance in energy transfer.
Differentiation between enthalpy in fluid (Hf) and gaseous (Hg) states, and the phase change enthalpy (Hfg).
The linear relationship between temperature and enthalpy in water up to the freezing point.
Calculation of energy required to convert water at 212 degrees Fahrenheit into steam.
Understanding saturated steam and its properties at 100% humidity.
How to find enthalpy values for saturated steam using temperature or pressure.
Organization of steam tables by temperature and the ease of finding enthalpy values.
The alternative organization of steam tables by pressure for quick reference.
Application of the heat flow equation in boiler examples to calculate required energy.
Example calculation of heating 100,000 pounds per hour of 60-degree water to 400-degree saturated steam.
Use of enthalpy values in calculating the energy change when steam condenses back into water.
Incorporate pressure aspect in steam calculations with an example involving 20.7 PSIA condensate.
Recap of the process for understanding steam tables, heat flow equations, and enthalpy applications.
Comparison of the process in SI units versus the traditional units used in the transcript.
Conclusion and invitation to the next section, emphasizing the practical applications of the discussed concepts.
Transcripts
Browse More Related Video

Introduction to Steam Tables
How to Use Steam Tables

How to use Steam Table - Easiest Way
How Much Thermal Energy Is Required To Heat Ice Into Steam - Heating Curve Chemistry Problems

Latent Heat of Fusion and Vaporization, Specific Heat Capacity & Calorimetry - Physics
Calorimetry Problems, Thermochemistry Practice, Specific Heat Capacity, Enthalpy Fusion, Chemistry
5.0 / 5 (0 votes)
Thanks for rating: