How to use Steam Table - Easiest Way
TLDRThis educational video script offers a comprehensive guide on utilizing steam tables for thermodynamic calculations. It explains key terms like saturation temperature and pressure, as well as concepts such as specific volume and enthalpy. The script walks through examples of interpreting data from both saturated and superheated steam tables, highlighting the process of finding thermodynamic properties and calculating the volume of a tank with a liquid-vapor mixture. It also demonstrates interpolation techniques for estimating missing values, providing a practical approach to steam table analysis.
Takeaways
- 🔍 Understanding Steam Tables: The script provides an educational guide on how to use and interpret steam tables, which are essential for students and professionals in thermodynamics.
- 🌡 Saturation Temperature: It is the temperature at which a liquid boils into vapor at a given pressure, with 100 degrees Celsius being the saturation temperature for water at one atmospheric pressure.
- 📉 Saturation Pressure: This is the pressure at which a liquid and its vapor can coexist in equilibrium at a specific temperature, such as one atmospheric pressure for water at 100 degrees Celsius.
- 📚 Basic Terminology: The script introduces key terms like 'EF' (property at saturated liquid state), 'AG' (property at saturated vapor state), and 'EFG' (change in property during phase change).
- 📈 Property Diagrams: The use of PV (Pressure-Volume) diagrams is explained to visualize the phase change process and the points representing saturated liquid and vapor states.
- 🍲 Dryness Fraction: Defined as the ratio of the mass of vapor to the total mass of the mixture, it varies between 0 (pure liquid) and 1 (pure vapor).
- 🔢 Specific Volume Calculation: The script explains how to calculate the specific volume of a mixture using the dryness fraction and the specific volumes of saturated liquid and vapor.
- 📊 Steam Table Types: Two main types of steam tables are discussed: the saturated steam table and the superheated steam table, each with its own method of interpretation.
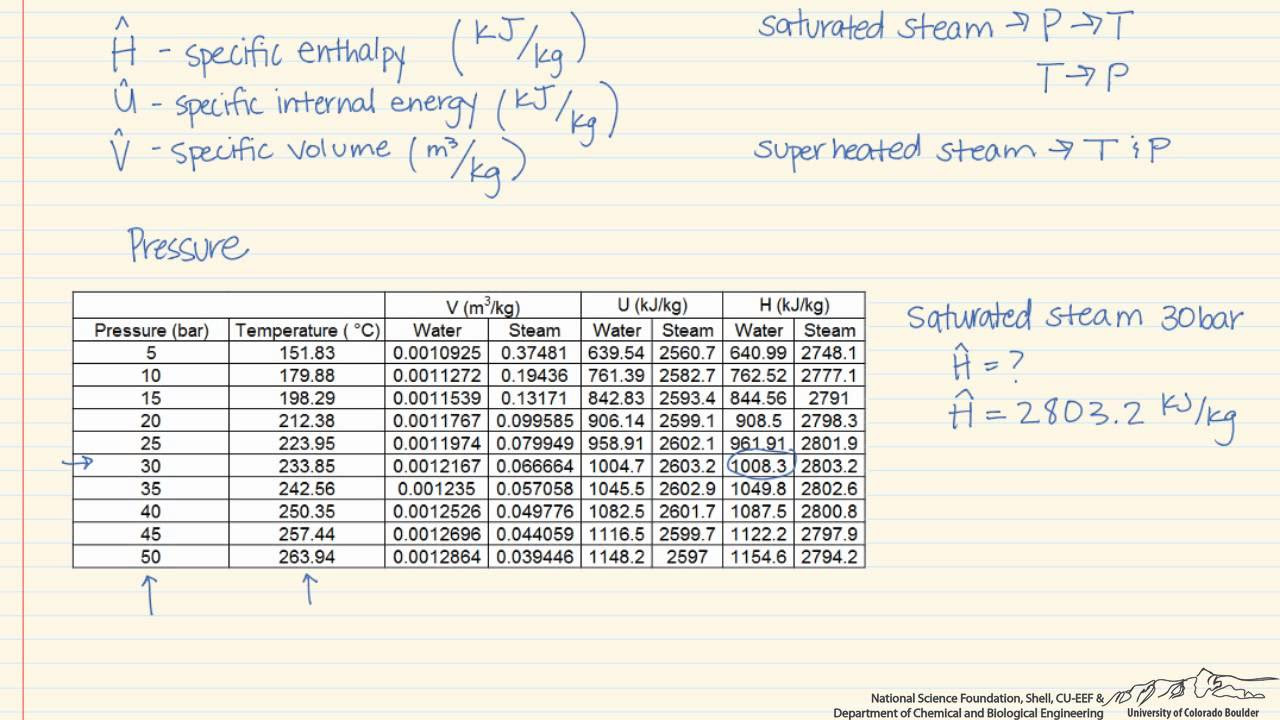
- 📋 Temperature-Based Steam Table: This table lists properties like specific volume, internal energy, enthalpy, and is used by starting with the temperature to find corresponding properties.
- 🔧 Pressure-Based Steam Table: This table is organized by pressure, listing saturation temperature and other properties, and is used to find properties at a given pressure.
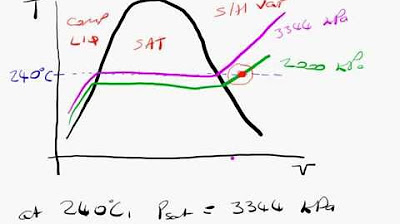
- 📝 Superheated Steam Table: Used for states where the temperature is above the saturation temperature, this table provides properties at various temperatures and pressures, with interpolation needed for unlisted specific volumes.
Q & A
What is the purpose of the video script?
-The purpose of the video script is to educate students on how to use and interpret data from a steam table, including understanding basic terminology and concepts related to thermodynamic properties of steam.
What is the saturation temperature?
-Saturation temperature is the temperature at which a liquid boils into vapor phase at a corresponding pressure. For water at one atmospheric pressure, the saturation temperature is 100 degrees Celsius.
What is meant by saturation pressure?
-Saturation pressure is the pressure at which a given liquid and its vapor can coexist in equilibrium at a given temperature. For water, one atmospheric pressure is the saturation pressure at 100 degrees Celsius.
What are the symbols used for properties at saturated liquid and vapor states?
-The symbol 'F' is used for properties at the saturated liquid state, and 'G' is used for properties at the saturated vapor state.
What is the definition of dryness fraction?
-Dryness fraction is defined as the ratio of the mass of vapor in the mixture to the total mass of the mixture, mathematically expressed as the mass of vapor divided by the mass of liquid plus mass of vapor.
How is the specific volume of a two-phase liquid-vapor system calculated?
-The specific volume of a two-phase liquid-vapor system can be calculated using the formula V = (1 - X) * VF + X * VG, where V is the specific volume, X is the dryness fraction, VF is the specific volume at saturated liquid state, and VG is the specific volume at saturated vapor state.
What are the two types of steam tables mentioned in the script?
-The two types of steam tables mentioned are the saturated steam table and the superheated steam table.
How can you find the specific volume of saturated vapor at a given temperature using a temperature-based steam table?
-In a temperature-based steam table, you look for the given temperature, then find the column that indicates saturated vapor and read the specific volume value for that state.
What is the significance of the equation V = VF + X * (VG - VF) in the context of the script?
-The equation V = VF + X * (VG - VF) is used to calculate the specific volume of a two-phase mixture, taking into account the dryness fraction X and the specific volumes of the saturated liquid (VF) and vapor (VG).
How does the script describe the process of using a pressure-based steam table?
-The script describes using a pressure-based steam table by first locating the given pressure in the first column, then finding the corresponding saturation temperature, and subsequently looking for the desired thermodynamic properties such as internal energy, enthalpy, etc.
What is the process of interpolation as described in the script for superheated steam tables?
-Interpolation in the context of superheated steam tables involves drawing a diagram with specific volume on the Y-axis and temperature on the X-axis, then connecting known data points and finding the unknown temperature or specific volume by drawing a straight line through the points and marking the desired value.
Outlines
🔍 Understanding Steam Tables and Terminology
This paragraph introduces the concept of steam tables and their importance in interpreting thermodynamic data. It defines key terms such as saturation temperature, saturation pressure, specific properties at saturated liquid (EF) and vapor (AG) states, and the change in property during phase change (EF G). The paragraph also explains the concept of dryness fraction, which is the ratio of the mass of vapor to the total mass in a mixture. The explanation includes diagrams and examples to illustrate the phase change process and how to calculate specific volumes and other properties using the steam table.
📚 Interpreting Data from Saturated and Superheated Steam Tables
This section delves into how to interpret data from steam tables, focusing on temperature-based and pressure-based saturated steam tables, as well as superheated steam tables. It explains the structure of these tables and provides examples of how to find specific volumes, internal energy, enthalpy, and entropy at given temperatures and pressures. The paragraph also demonstrates how to calculate the volume of a rigid tank containing a mixture of liquid and vapor water using the dryness fraction and specific volumes from the steam table.
🔧 Practical Examples of Using Steam Tables for Thermodynamic Calculations
Building on the previous explanation, this paragraph provides practical examples of using steam tables for thermodynamic calculations. It shows how to determine the internal energy of saturated liquid at a given pressure and saturation temperature, and how to interpret data from superheated steam tables. The paragraph also illustrates the process of interpolation when exact values are not available in the table, using a step-by-step example to find the temperature corresponding to a specific volume at a given pressure.
👍 Conclusion and Call for Engagement
The final paragraph concludes the video script by inviting viewers to like, share, and comment if they have any queries. It serves as a call to action, encouraging viewer engagement and providing a platform for further discussion and clarification of concepts covered in the video.
Mindmap
Keywords
💡Steam Table
💡Saturation Temperature
💡Saturation Pressure
💡Specific Volume
💡Enthalpy
💡Internal Energy
💡Dryness Fraction
💡Saturated Liquid
💡Saturated Vapor
💡Superheated Steam Table
💡Interpolation
Highlights
Introduction to the use and interpretation of steam tables for students facing difficulties.
Basic terminology associated with steam tables: saturation temperature, saturation pressure, specific properties at saturated states, and dryness fraction.
Saturation temperature is the temperature at which a liquid boils into vapor at a given pressure.
Saturation pressure is the equilibrium pressure between liquid and vapor at a given temperature.
Explanation of specific properties at saturated liquid (AF) and vapor (AG) states, and the change in property (AFG) during phase change.
Dryness fraction defined as the ratio of mass of vapor to total mass in a mixture.
Mathematical representation of dryness fraction and its significance in the phase change process.
Illustration of dryness fraction on a PV diagram with examples of liquid and vapor states.
Calculation of specific volume in a liquid-vapor system using the dryness fraction.
Thermodynamic properties evaluation using steam tables: specific volume, internal energy, enthalpy, and entropy.
Types of steam tables: saturated and superheated, with temperature or pressure as the basis.
Interpretation of data from temperature-based steam tables, including specific volume and enthalpy of saturated states.
Example problem solving using steam tables: determining the volume of a rigid tank containing a liquid-vapor mixture.
Pressure-based steam tables explained, focusing on the relationship between pressure, temperature, and thermodynamic properties.
Superheated steam tables and their limitations in providing values below saturation temperature.
Interpolation method demonstrated for determining temperature at a specific volume not directly listed in the steam table.
Practical examples of using steam tables for determining specific enthalpy and temperature in various states of water.
Encouragement for viewers to like, share, and comment on the video for further queries.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: