How to Use Steam Tables
TLDRThis screencast tutorial introduces the use of steam tables, focusing on properties like specific enthalpy, internal energy, and volume for steam. It distinguishes between saturated and superheated steam, explaining how to determine temperature or pressure for saturated steam and requiring both for superheated steam. The script guides through finding properties at specific pressures and temperatures, illustrating with examples like 30 bar saturated steam and 60 bar superheated steam at 500°C.
Takeaways
- 📚 Steam tables are essential tools for listing properties of steam, such as specific enthalpy, specific internal energy, and specific volume.
- 🔍 The term 'specific' refers to properties per unit mass or unit moles, typically expressed in kJ/kg for enthalpy and internal energy, and in m³/kg for volume.
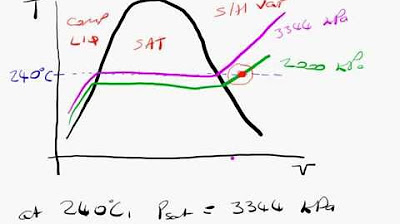
- 🌡 Saturated steam tables link pressure and temperature directly, meaning knowing one fixes the other due to the equilibrium between steam and water.
- 📊 To find properties of saturated steam, one can use tables organized by pressure or temperature, each providing corresponding values for temperature, specific volume, internal energy, and enthalpy.
- 🔑 At a given pressure, such as 30 bar, the script provides a method to find the specific enthalpy of steam, which in this case is 2803.2 kJ/kg.
- 💧 For saturated water, the enthalpy and specific volume can also be determined from the steam tables, as demonstrated with an example at 45 bar.
- 🌟 Superheated steam requires both pressure and temperature to determine its properties because it is beyond the vapor-liquid equilibrium line.
- 🔍 When dealing with superheated steam at a specific pressure and temperature, such as 60 bar and 500°C, the script explains how to find properties like specific enthalpy, internal energy, and volume.
- 📘 Understanding steam tables allows for solving mass and energy balances in various engineering applications that utilize steam.
- 📈 The script demonstrates practical examples of how to use steam tables to find specific properties of steam at different pressures and temperatures.
- 🛠 Mastery of steam tables is crucial for engineers and scientists working in fields where steam is a critical component of processes and systems.
Q & A
What are the specific properties of steam that the screencast focuses on?
-The screencast focuses on specific enthalpy, specific internal energy, and specific volume of steam.
What does the term 'specific' mean in the context of steam tables?
-In the context of steam tables, 'specific' refers to the properties per unit mass or per unit mole.
What units are used to measure enthalpy and internal energy in the steam tables discussed in the screencast?
-In the steam tables discussed, enthalpy and internal energy are measured in kilojoules per kilogram (kJ/kg).
How is specific volume measured in the steam tables?
-Specific volume in the steam tables is measured in cubic meters per kilogram (m³/kg).
What is the significance of saturated steam in the context of steam tables?
-Saturated steam refers to a state where steam and water coexist in equilibrium. Once the pressure is known, the temperature is automatically fixed, and vice versa.
What is the difference between using steam tables by pressure and by temperature?
-Using steam tables by pressure allows you to find properties when you know the pressure, and using them by temperature allows you to find properties when you know the temperature.
How do you find the specific enthalpy of saturated steam at 30 bar using the steam tables?
-You look at the table under 30 bar, and find that the specific enthalpy of saturated steam at this pressure is 2803.2 kJ/kg.
What is the specific volume of saturated water at 45 bar according to the steam tables?
-The specific volume of saturated water at 45 bar is 0.0012696 m³/kg.
Why do you need both pressure and temperature to find properties of superheated steam?
-Superheated steam is beyond the vapor-liquid equilibrium line, so both pressure and temperature are needed to determine its properties.
How can you use steam tables to solve mass and energy balances in steam systems?
-Once you are familiar with steam tables, you can use the properties listed to calculate mass and energy balances in systems that utilize steam.
What are the three parts of the steam tables that are usually used according to the screencast?
-The three parts of the steam tables usually used are those related to saturated steam, properties by pressure, and properties by temperature.
Outlines
💧 Understanding Saturated Steam Properties
This paragraph introduces the concept of steam tables, focusing on specific enthalpy, internal energy, and volume of steam. It explains that these properties are measured per unit mass and are typically presented in kJ/kg for enthalpy and internal energy, and in m³/kg for specific volume. The paragraph distinguishes between saturated steam, where steam and water are in equilibrium at a given pressure or temperature, and superheated steam, which requires both pressure and temperature to define its properties. The speaker demonstrates how to use steam tables to find the specific enthalpy of saturated steam at 30 bar, yielding a value of 2803.2 kJ/kg, and the specific volume of saturated water at 45 bar, which is 0.0012696 m³/kg.
🔥 Superheated Steam and Property Determination
Building on the previous discussion, this paragraph delves into the properties of superheated steam, emphasizing the necessity of knowing both pressure and temperature to determine its specific properties. The speaker illustrates the use of steam tables for superheated steam by providing an example at 60 bar and 500 degrees Celsius, where the specific enthalpy is 3423.1 kJ/kg, the specific internal energy is 3083.1 kJ/kg, and the specific volume is 0.056671 m³/kg. The paragraph concludes by suggesting that once one is familiar with steam tables, they can apply the properties to solve mass and energy balances involving steam.
Mindmap
Keywords
💡Steam Tables
💡Specific Enthalpy
💡Specific Internal Energy
💡Specific Volume
💡Saturated Steam
💡Pressure
💡Temperature
💡Superheated Steam
💡Vapor-Liquid Equilibrium Line
💡Mass and Energy Balances
Highlights
Introduction to the use of steam tables for listing properties of steam.
Focus on specific enthalpy, specific internal energy, and specific volume in steam tables.
Explanation of the term 'specific' in the context of steam tables.
Units of measurement for enthalpy and internal energy in kJ per kg.
Units for specific volume in meters cubed per kg.
Differentiation between three parts of steam tables: saturated steam, water properties, and superheated steam.
Saturated steam properties are determined by pressure or temperature.
Superheated steam requires both pressure and temperature for property determination.
How to use steam tables for saturated steam by pressure.
Example of finding specific enthalpy for saturated steam at 30 bar.
Finding specific volume for saturated water at 45 bar.
Use of steam tables for properties under temperature for superheated steam.
Example of finding specific enthalpy, internal energy, and volume for superheated steam at 60 bar and 500 degrees Celsius.
Importance of knowing both pressure and temperature for superheated steam properties.
Practical application of steam table properties for solving mass and energy balances.
Emphasis on the value of mastering steam tables for engineering calculations.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: