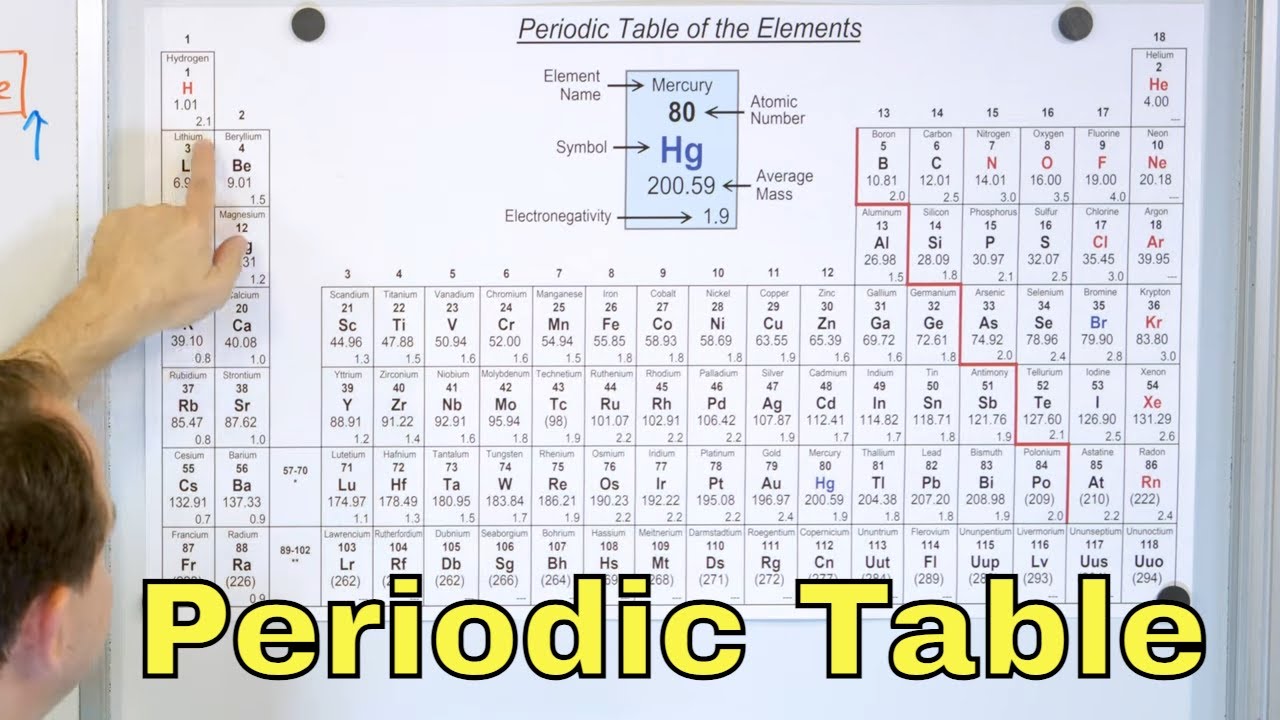
Groups of the Periodic Table
TLDRThis script delves into the electron configurations of elements, focusing on the quest for stability with eight electrons in the outermost shell. It explains the periodic table's groupings, highlighting the reactivity of alkali and alkaline earth metals due to their electron-donating tendencies. The video also touches on the transition metals' electron-sharing properties, which contribute to their conductivity, and concludes with the noble gases' stability and unreactivity, exemplified by helium's use in balloons for safety.
Takeaways
- 🧲 Atoms prefer to have eight electrons in their outermost shell for stability.
- 📊 The periodic table is organized by groups, with elements in the same group sharing the same number of valence electrons.
- 🌟 Noble gases are a special group with full outer shells, making them very stable and unreactive.
- 🔍 Elements on the left side of the periodic table tend to lose electrons easily, while those on the right gain them.
- 🌱 Alkali metals, such as lithium, sodium, and potassium, are highly reactive and rarely found in their elemental state.
- 💎 Alkaline earth metals, like magnesium and calcium, are less reactive than alkali metals but still want to lose two electrons to achieve stability.
- 🌈 Transition metals fill the d subshell and are known for their ability to form multiple oxidation states.
- 🔌 Metals, especially those with filled d orbitals, are good conductors of electricity due to the presence of surplus electrons.
- 🏆 Silver is the best conductor of electricity among all elements.
- 💡 The reactivity of metals decreases as you move down a group because the valence electrons are further from the nucleus.
- 🚫 Nonmetals, especially halogens, are more likely to gain electrons to achieve a stable electron configuration.
Q & A
Why do atoms want to have eight electrons in their outermost shell?
-Atoms desire to have eight electrons in their outermost shell because this configuration is the most stable. It is determined by observing the behavior of elements and their tendency to achieve a full valence shell.
What is a group in the periodic table?
-A group in the periodic table refers to a column. Each column represents a group of elements that share similar chemical properties due to having the same number of valence electrons.
Why are the elements in the noble gas group considered stable?
-Elements in the noble gas group are stable because they have a full outer shell of electrons, typically eight, which makes them chemically inert and less likely to react with other elements.
What is the significance of having the same number of valence electrons in a group?
-Having the same number of valence electrons in a group means that the elements in that group will exhibit similar chemical properties and reactivity. This is because the valence electrons are the ones that participate in chemical bonding.
Why are alkali metals more reactive than alkaline earth metals?
-Alkali metals are more reactive than alkaline earth metals because they have only one electron in their outermost shell, which they readily give away to achieve a stable electron configuration. Alkaline earth metals, having two electrons in their outermost shell, are less likely to give away electrons and thus are less reactive.
What is the elemental state of an element?
-The elemental state of an element refers to the form in which an element exists without being combined with any other element. For example, lithium in its elemental state would be just lithium atoms, not bonded with any other elements.
How do transition metals achieve stability?
-Transition metals achieve stability by filling their d subshells. Even if they lose their 4s electrons, they still have a 'reserve' of electrons in their d subshell from the previous shell, which can replace the lost electrons, maintaining stability.
Why do metals conduct electricity well?
-Metals conduct electricity well because they have a 'sea' of loosely held valence electrons that can move freely. These electrons are not tightly bound to any single atom, allowing them to move easily and thus facilitating the flow of electric current.
What is the difference between transition metals and other metals in terms of electron configuration?
-Transition metals are distinguished by their partially filled d subshells, which they are in the process of filling. Other metals, like the alkali and alkaline earth metals, have simpler electron configurations with fewer valence electrons that they are more likely to lose or share.
Why are noble gases unreactive?
-Noble gases are unreactive because they have a full outer shell of electrons (eight for most, two for helium). This full valence shell makes them chemically stable and disinclined to participate in chemical reactions.
Outlines
🌌 Electron Configuration and the Periodic Table
This paragraph discusses the electron configuration of atoms, particularly the preference for eight electrons in the outermost shell for stability. It introduces the concept of groups in the periodic table and highlights the noble gases as a special group. The paragraph explains how elements in a group share the same number of valence electrons and how this affects their chemical behavior, with alkali metals being eager to give away electrons and noble gases being stable due to their electron configuration.
🔬 Reactivity Trends and Metal Characteristics
The second paragraph delves into the reactivity trends of elements across the periodic table, focusing on metals and their tendency to give away electrons. It explains the formation of metallic bonds in metals like aluminum, which contribute to their conductivity. The paragraph also touches on the transition metals, which are filling their d subshells and have a surplus of electrons, making them good conductors. It contrasts these with nonmetals, which are more likely to gain electrons, and discusses the unique stability of noble gases.
🎈 Noble Gases: Stability and Reactivity
The final paragraph focuses on the noble gases, emphasizing their stability due to having eight electrons in their outermost shell, with the exception of helium, which has two. It describes how this electron configuration makes noble gases unreactive, contrasting them with other elements that are more prone to chemical reactions. The paragraph also provides a historical context, mentioning the use of hydrogen in the Hindenburg and the preference for helium in balloons today due to its inert nature.
Mindmap
Keywords
💡Valence electrons
💡Noble gases
💡Alkali metals
💡Alkaline earth metals
💡Transition metals
💡Electron configuration
💡Periodic table
💡Metallic bonds
💡Halogens
💡Conductivity
Highlights
Atoms desire to have eight electrons in their outermost shell for stability.
A group in the periodic table is a column, sharing the same number of valence electrons.
Noble gases are a special group with elements having full outer shells, making them unreactive.
Alkali metals, like lithium, have one valence electron and are very reactive, often found in ionic compounds.
Hydrogen is not typically considered an alkali metal due to its different properties.
Alkaline earth metals have two valence electrons and are less reactive than alkali metals.
Transition metals fill the d subshell, maintaining two valence electrons, and are considered stable.
Metals tend to give away electrons easily, especially those with fewer valence electrons.
Alkali metals are rarely found in their elemental state due to their reactivity.
Alkaline earth metals are closer to achieving a stable electron configuration, making them less reactive.
Transition metals have a 'deep bench' of electrons, allowing them to lose valence electrons without destabilizing.
Metals form metallic bonds by sharing electrons, which is why they are good conductors.
Silver is the best conductor of electricity, but copper is more commonly used due to availability.
Elements with filled d orbitals are more stable and less likely to participate in reactions.
Nonmetals tend to gain electrons to achieve a stable electron configuration.
Halogens are highly reactive, especially with alkali metals, and are used in applications like halogen lamps.
Noble gases are inert due to their complete outer electron shells, making them unreactive.
Helium, despite being a noble gas, only has two electrons in its outer shell, unlike other noble gases.
The noble gas helium is used in balloons due to its unreactive nature, making it safer than hydrogen.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: