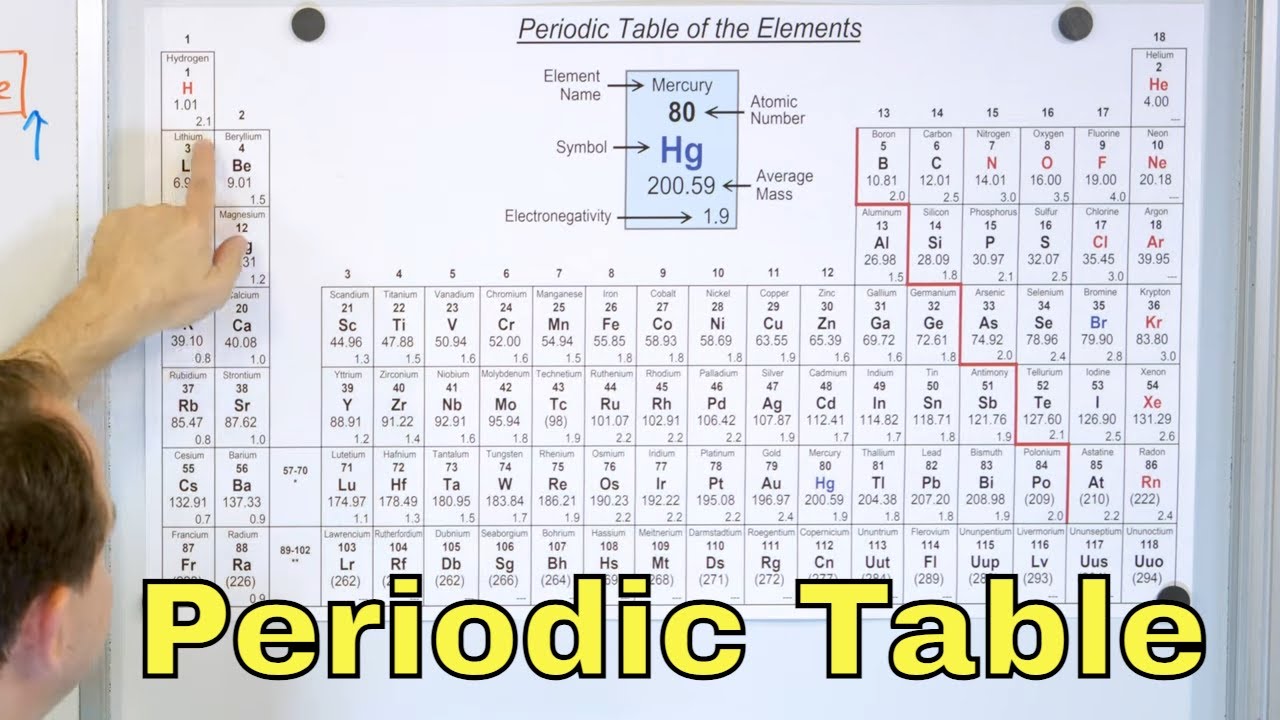
Valence Electrons
TLDRThis script delves into the concept of electron configurations and their significance in the periodic table's organization. It explains how elements' reactions can be predicted based on their valence electrons, emphasizing the goal of achieving a stable configuration with eight electrons in the outermost shell. The video discusses the electron configurations of various elements like lithium, iron, and selenium, illustrating the 'noble gas' notation for shorthand representation. It also touches on the reactivity of alkali metals and halogens due to their tendencies to lose or gain electrons to reach stability.
Takeaways
- 🔬 Lithium's electron configuration is 1s² 2s¹, which can also be written as [He] 2s¹.
- 🧪 Iron's electron configuration can be written as [Ar] 4s² 3d⁶, highlighting the backfilling of the d subshell.
- 🧩 Elements in the d-block of the periodic table backfill the previous shell's d orbitals after filling the s orbital of their current period.
- 📊 The number of valence electrons is crucial for determining the reactivity of an element.
- 🧮 Elements in the same group (column) on the periodic table have the same number of valence electrons, leading to similar chemical properties.
- 🔋 Atoms aim to have eight electrons in their outermost shell (the octet rule) for stability, except for hydrogen and helium.
- ⚛️ Alkali metals (group 1) are highly reactive because they have one electron in their outermost shell and readily lose it to achieve a stable configuration.
- 🌟 Halogens (group 17) are also highly reactive as they have seven electrons in their outermost shell and readily gain an electron to complete their octet.
- 💡 Electron configurations can be abbreviated by noting the previous noble gas configuration followed by the additional electrons.
- 🔍 Valence electrons, or the electrons in the outermost shell, are primarily responsible for an element's chemical reactions.
Q & A
What is the electron configuration of lithium?
-The electron configuration of lithium is 1s² 2s¹, which is the same as helium's configuration plus an additional 2s¹ electron.
How can you quickly determine the electron configuration of an element like iron?
-You can quickly determine iron's electron configuration by starting with argon's configuration (which has the same number of electrons) and then adding 4s² 3d⁶.
Why does the electron configuration of elements in the d-block involve backfilling the previous shell?
-Elements in the d-block backfill the previous shell because as the atom grows larger, there are more spaces between the previous orbitals, making it energetically favorable to fill these spaces before moving on to the next shell.
What is the electron configuration for the outermost shell of iron?
-The outermost shell of iron has two electrons, which are in the 4s² configuration.
How many valence electrons does a typical element in the first group of the periodic table have?
-A typical element in the first group of the periodic table has one valence electron in its outermost shell.
What is the significance of the number eight in the context of electron configurations?
-The number eight is significant because atoms tend to be most stable when they have eight electrons in their outermost shell, which is known as the octet rule.
Why do alkali metals tend to lose electrons easily?
-Alkali metals tend to lose electrons easily because they have one electron in their outermost shell and losing this electron allows them to achieve a stable electron configuration similar to that of a noble gas.
What is the electron configuration of an element in the p-block of the periodic table?
-An element in the p-block of the periodic table will have its valence electrons in the p subshell of the outermost shell, typically with three to eight electrons depending on the group number.
How does the electron configuration of an atom influence its reactivity?
-The electron configuration of an atom, particularly the number of valence electrons, influences its reactivity. Atoms with one or two valence electrons tend to lose electrons to achieve a stable configuration, while atoms with seven valence electrons tend to gain an electron to complete their outer shell.
Why does hydrogen have a different preference for electron configuration compared to other alkali metals?
-Hydrogen has a different preference because it has only one electron in its single shell and is satisfied with having two electrons to complete its shell, unlike other alkali metals which have one electron in their outermost shell and prefer to lose it to achieve stability.
What is the electron configuration for selenium (Se)?
-Selenium's electron configuration is the same as krypton's, followed by 4d¹⁰ 5s², which gives it four valence electrons in its outermost shell.
Outlines
🧠 Electron Configurations and Periodic Table Grouping
This paragraph delves into the concept of electron configurations and how they can be used to categorize elements on the periodic table. The explanation begins with the electron configuration of lithium, simplifying it by comparing it to helium plus an additional 2s1 electron. The script then moves on to iron, using the electron configuration of argon as a base and adding the specific electrons for iron, including the backfilling of the 3d subshell. The paragraph also addresses the question of why atoms backfill orbitals, offering an intuitive explanation related to the increasing space between orbitals as the atom grows larger. The focus is on understanding the electron configurations to predict how elements might react with others.
🌌 Understanding Valence Electrons and Periodic Trends
The second paragraph continues the discussion on electron configurations but shifts the focus to valence electrons—the electrons in the outermost shell—and their significance in chemical reactions. It explains how different groups in the periodic table have patterns of valence electrons, such as the first group having one electron and the d-block elements having two. The paragraph also introduces the concept of backfilling in the d-block and p-block, using selenium (Sn) as an example to illustrate the electron configuration and valence electrons. It emphasizes the importance of valence electrons in determining how elements will react with other atoms or electrons, setting the stage for understanding chemical reactivity.
🔬 Atomic Stability and the Quest for Eight Valence Electrons
This paragraph explores the idea that atoms strive for a stable configuration with eight electrons in their outermost shell, which is considered the most stable state. It discusses how atoms with fewer than eight valence electrons, such as potassium with one and chlorine with seven, will naturally react to achieve this stable configuration. Potassium, for instance, will tend to lose its outer electron, while chlorine will want to gain one, leading to a reaction where potassium transfers its valence electron to chlorine. The paragraph highlights the reactivity of alkali metals, which readily give away electrons, and halogens, which readily accept them, to achieve a stable electron configuration similar to that of noble gases like argon. It also notes that hydrogen and helium are exceptions to this rule, being satisfied with two electrons due to having only one shell.
🌟 Metallic Nature and Chemical Reactivity
The final paragraph touches on the concept of metallic nature in chemistry, which is related to how eager an element is to lose electrons. It summarizes the previous discussion by emphasizing the reactivity of alkali metals, which are inclined to donate their valence electrons to achieve a stable electron configuration. The paragraph concludes by indicating that the next video will further explore the groups in the periodic table and the trends that can be observed from them, hinting at a deeper dive into the periodic trends and the chemical properties of elements.
Mindmap
Keywords
💡Electron Configuration
💡Valence Electrons
💡Periodic Table
💡Alkali Metals
💡Halogens
💡Noble Gases
💡Electron Shells
💡d-Block
💡Stable Configuration
💡Chemical Reactivity
💡Periodic Trends
Highlights
Introduction to determining electron configurations and their role in grouping elements on the periodic table.
Practice with lithium's electron configuration, illustrating the method of adding an electron to helium's configuration.
Explanation of iron's electron configuration using argon's configuration as a reference.
Discussion on the backfilling of the d subshell in the d-block of the periodic table.
Intuitive explanation of why atoms backfill the d orbital, relating to the growth of the atom and space between orbitals.
Clarification that the outermost shell determines the reactivity of an element.
Identification of lithium having one electron in its outermost shell.
Iron having two electrons in its outermost shell, typical for elements in the d-block.
Generalization that elements in the d-block fill the outermost shell with two electrons.
Introduction to the concept of valence electrons and their significance in chemical reactions.
Explanation of how the p-block elements have three valence electrons in their outermost shell.
Demonstration of selenium's electron configuration and its valence electrons.
Discussion on the desire of atoms to have eight electrons in their outermost shell for stability.
Potential chemical reaction between potassium and chlorine based on their valence electrons.
Description of alkali metals' tendency to give away electrons and their high reactivity.
Differentiation between hydrogen and other alkali metals in terms of electron giving away tendency.
Introduction to the concept of metallic nature in relation to electron giving away tendency.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: