AP Chemistry Unit 1 Review: Atomic Structure and Properties!!
TLDRThe video script is an engaging and comprehensive review of AP Chemistry, focusing on key concepts such as atomic structures, moles, molar mass, isotopes, and the periodic trends. The presenter, Cara, uses a conversational tone to explain complex topics like the significance of Avogadro's number, the calculation of average atomic mass, and the principles of mass spectrometry. She also delves into Dalton's atomic theory, chemical bonding, and electron configurations, providing mnemonics and tricks to help students remember the information. The script covers the periodic table's organization, including groups like alkali metals, alkaline earth metals, transition metals, and noble gases, and discusses trends in atomic radius, ionization energy, electron affinity, and electronegativity. Cara's approach is student-friendly, offering a mix of humor and clear explanations to make chemistry more accessible.
Takeaways
- 🧪 The concept of a mole in chemistry refers to 6.02 x 10^23 units, which can be atoms, molecules, or ions, and is used to convert between grams and atomic mass units.
- 📊 Molar mass is the mass of one mole of a substance, and it's calculated by multiplying the atomic mass unit by the number of moles.
- 🔬 Isotopes are variants of a chemical element that have the same number of protons but different numbers of neutrons, leading to different masses.
- 🧬 Dalton's atomic theory states that atoms are the smallest units of matter, conserved in chemical reactions, and compounds have a characteristic ratio of atoms.
- ⚛️ Ions are formed when atoms gain or lose electrons, resulting in a net charge, and can lead to the formation of ionic or covalent bonds.
- 🔋 Electron configurations describe the arrangement of electrons in an atom's orbitals, following the Pauli exclusion principle and energy level rules.
- 🧲 Unpaired electrons in the valence shell can result in paramagnetism, where the atom is attracted to a magnetic field, versus diamagnetism where the atom repels the magnetic field.
- 🌟 Photoelectron spectroscopy involves the ejection of electrons from atoms by shining light on them, providing data on electron energy levels and configurations.
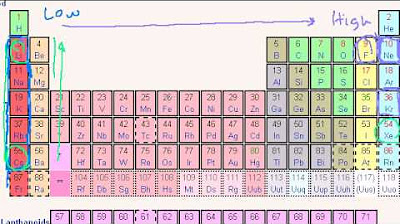
- 📉 Atomic radius generally decreases across a period due to increased nuclear charge attracting electrons more strongly, and increases down a group due to additional electron shells.
- ⚡ Ionization energy is the energy required to remove an electron, which tends to be lower for metals and higher for nonmetals, with Francium having the lowest ionization energy.
- 🔗 Electronegativity measures an atom's ability to attract electrons in a covalent bond, with nonmetals like Fluorine being the most electronegative.
- 🌌 Metallicity refers to the tendency of an element to lose electrons and form positive ions, with elements like Francium being highly metallic.
Q & A
What is the significance of Avogadro's number in chemistry?
-Avogadro's number, which is approximately 6.02 x 10^23, signifies the number of atoms, molecules, or ions in one mole of a substance. It is crucial for converting between moles, atomic mass units, and the actual number of particles in a sample.
How does mass spectrometry work, and what information can it provide about isotopes?
-Mass spectrometry involves ionizing atoms or molecules and then separating them based on their mass-to-charge ratio. It can provide information about the relative abundance of different isotopes in a sample, as well as their individual masses.
What is Dalton's Law of Constant Composition, and how does it relate to chemical formulas?
-Dalton's Law of Constant Composition states that a given compound will always be composed of the same ratio of elements by mass. This principle allows chemists to write chemical formulas that represent the composition of compounds.
How does the periodic table help in understanding electron configurations?
-The periodic table is organized to reflect the electron configurations of elements. Elements in the same group have similar valence electron configurations, which can be used to predict their chemical properties and reactivity.
What is an ionic bond, and how does it differ from a covalent bond?
-An ionic bond is a type of chemical bond formed by the electrostatic attraction between oppositely charged ions, typically a metal and a nonmetal. This differs from a covalent bond, where atoms share one or more pairs of electrons, usually between two nonmetals.
How does the number of unpaired electrons in an atom's valence shell affect its properties?
-The number of unpaired electrons in an atom's valence shell can influence its reactivity and the types of chemical bonds it can form. Atoms with unpaired electrons tend to be more reactive and can participate in various types of chemical reactions.
What is the relationship between an atom's atomic radius and its position in the periodic table?
-As you move down a group in the periodic table, the atomic radius generally increases due to the addition of electron shells. Conversely, as you move across a period from left to right, the atomic radius decreases due to increased nuclear charge attracting the electrons more strongly.
What is the significance of electronegativity in determining the type of chemical bond formed between two atoms?
-Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A large difference in electronegativity between two atoms typically results in an ionic bond, while a smaller difference or similar electronegativity values lead to covalent bonding.
How does the effective nuclear charge influence the behavior of an atom's valence electrons?
-The effective nuclear charge is the net positive charge experienced by an electron in a shell. It affects the behavior of valence electrons by pulling them closer to the nucleus, which can influence the atom's reactivity and the types of bonds it forms.
What is photoelectron spectroscopy, and how can it be used to determine electron configurations?
-Photoelectron spectroscopy is a technique that measures the kinetic energy of electrons ejected from a material after being excited by light. It can be used to determine the electron configuration of an atom by analyzing the energy levels from which the electrons are emitted.
What are the trends in ionization energy and electron affinity as you move across a period and down a group in the periodic table?
-Generally, ionization energy increases across a period and decreases down a group. This is because atoms with more protons (across a period) have a stronger attraction for their electrons, while those with additional electron shells (down a group) have increased distance and shielding effects. Electron affinity usually increases across a period as atoms become more electronegative, but it can vary down a group due to changes in atomic size and electron shielding.
Outlines
🌟 Introduction to AP Chemistry Review
The video begins with the host, Cara, introducing the AP Chemistry review session. She candidly admits that chemistry is one of her weaker subjects but is determined to cover Unit 1 of the AP Chemistry curriculum, which focuses on atomic structures and properties. The first topic of discussion is moles and molar mass, with Cara emphasizing the importance of understanding Avogadro's number, which is 6.02 times 10 to the 23rd. She explains that a mole is a unit that allows for the conversion between atomic mass units and grams, and she provides examples to illustrate how to calculate the number of moles and the number of molecules in a given mass of an element.
🔬 Understanding Isotopes and Atomic Mass
Cara delves into isotopes, explaining that they are atoms of the same element with different numbers of neutrons, resulting in different masses. She discusses how the average atomic mass of an element is calculated by taking a weighted average of its isotopes, using carbon as an example. The video also touches on mass spectrometry, a technique used to determine the mass-to-charge ratio of ions, which is key to identifying isotopes. Cara explains the process of mass spectrometry and how it can be used to calculate the relative abundance of isotopes.
🔍 Dalton's Atomic Theory and Chemical Formulas
The video moves on to discuss Dalton's atomic theory, which posits that atoms are the smallest units of matter, that mass is conserved in chemical reactions, and that compounds have a characteristic ratio of atoms. Cara talks about different types of substances, including elements, compounds, mixtures, and solutions. She also covers how to write chemical formulas for compounds and introduces the concept of empirical formulas, which are simplest whole-number ratios of atoms in a compound.
🔋 Ions, Ionic Bonds, and Covalent Bonds
Cara explains the concept of ions, which are atoms that have gained or lost electrons, resulting in a positive or negative charge. She differentiates between anions and cations and discusses how ionic bonds form between atoms with opposite charges. The video also covers covalent bonds, where electrons are shared between atoms. Cara provides examples of how to name ionic compounds and emphasizes the importance of knowing polyatomic ions. She also briefly touches on electron configurations and the structure of atoms.
⚛️ Electron Configurations and Quantum Numbers
The video dives into electron configurations, explaining the role of quantum numbers in determining where electrons reside in an atom's orbitals. Cara describes the four quantum numbers and how they define an electron's unique position and spin. She discusses the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers. The video also explains how to use the periodic table to determine electron configurations and the exceptions to the general rules, such as those involving the d-block and f-block elements.
🌈 Photoelectron Spectroscopy and Periodic Trends
Cara introduces photoelectron spectroscopy, a technique that measures the kinetic energy of electrons ejected from a material exposed to light. She explains how this technique can be used to determine electron configurations. The video concludes with a discussion of periodic trends, such as atomic radius, ionization energy, electron affinity, and metallicity. Cara explains how these properties change across periods and groups in the periodic table and provides mnemonic devices to help remember these trends.
📝 Conclusion and Viewer Engagement
In the final paragraph, Cara thanks viewers for watching and invites them to provide feedback or suggestions for future content. She expresses her willingness to adapt her teaching style based on viewer input and encourages viewers to like and subscribe for more educational content. The video ends with a promise to cover more topics in subsequent sessions.
Mindmap
Keywords
💡Mole
💡Molar Mass
💡Isotope
💡Mass Spectrometry
💡Dalton's Atomic Theory
💡Chemical Formula
💡Ionic Bond
💡Electron Configuration
💡Unpaired Electrons
💡Periodic Trends
💡Photoelectron Spectroscopy
Highlights
Introduction to AP Chemistry review focusing on Unit 1: Atomic Structures and Properties.
Explanation of moles and molar mass, emphasizing the importance of Avogadro's number.
Conversion between atomic mass units and grams using moles as a桥梁 (bridge).
Understanding isotopes as atoms with the same number of protons but different numbers of neutrons.
Calculating average atomic mass by taking a weighted average of isotopes' masses.
Mass spectrometry's role in determining the mass-to-charge ratio of ions and its application in identifying isotopes.
Dalton's atomic theory and the law of constant composition in chemistry.
Writing chemical formulas and converting them to empirical formulas.
Understanding ionic and covalent bonds, and the formation of ionic compounds.
Naming conventions for ionic compounds, especially with transition metals.
Electron configurations following the Pauli exclusion principle and the ordering of orbitals.
Exceptions to electron configurations in d-block elements like chromium and copper.
Determining the number of unpaired electrons in an atom and its relation to element properties.
Photoelectron spectroscopy and its use in identifying electron configurations.
Periodic trends in atomic radius, ionization energy, electron affinity, and metallic character.
The impact of electronegativity on bond types, distinguishing between covalent, polar covalent, and ionic bonds.
A request for feedback on the presentation method and content coverage for future reviews.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: