Periodic Table of Elements - Element Classes
TLDRIn this educational video, Mr. Melons explores the diverse classes of elements on the periodic table, detailing the properties and characteristics of metals, nonmetals, and metalloids. He delves into the physical and chemical attributes of alkali and alkaline earth metals, transition metals, halogens, and noble gases. The video also highlights the unique properties of lanthanides and actinides, offering viewers a comprehensive understanding of the periodic table's structure and the behavior of its elements.
Takeaways
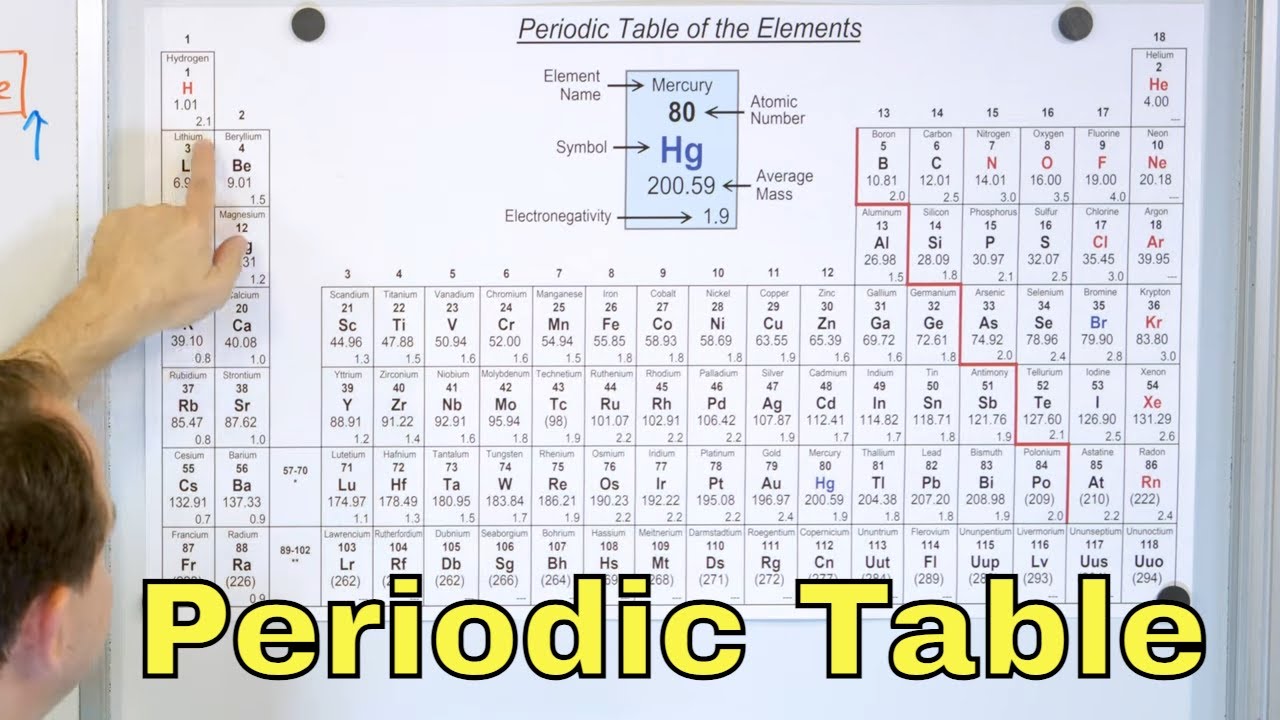
- 🔍 The periodic table categorizes elements into metals, nonmetals, and metalloids based on their properties relative to a 'stair-step line'.
- 🌟 Metals are typically hard, shiny, malleable, ductile, and have good electrical and thermal conductivity, with most being solids at room temperature except for mercury.
- 💎 Nonmetals are brittle, poor conductors of heat and electricity, making them good insulators, and often lack metallic attributes such as shininess and high density.
- 🌐 Metalloids or semimetals have properties intermediate between metals and nonmetals, including being electrical semiconductors and having electronegativity values between the two.
- 🧪 Alkali metals (Group 1, excluding hydrogen) are highly reactive, have one valence electron, and are soft with low densities and melting points.
- 🏔️ Alkaline earth metals (Group 2) are also reactive, form strong bases in water, and have two valence electrons, with shiny, silvery-white appearance and low boiling and melting points.
- 🌈 Transition metals (Groups 3-12) form colored compounds, are less reactive than alkali and alkaline earth metals, and have high melting points and varying oxidation states.
- 🧂 Halogens (Group 17) are nonmetals that react with metals to form salts, have seven valence electrons, and form diatomic molecules, being highly reactive and never found alone in nature.
- 💨 Noble gases (Group 18) are chemically inert and stable, typically colorless and odorless, existing as monatomic gases with a completely filled outer energy level.
- 🌌 Lanthanides (57-71 on the periodic table) are rare earth metals with high melting and boiling points, reactive, and often silvery white in appearance.
- ⚛️ Actinides (89-103 on the periodic table) are radioactive, tarnish in air, and include man-made elements not found naturally, with properties like high density and electropositivity.
Q & A
What are the general physical properties of metals according to the video?
-Metals are typically hard, shiny, malleable, ductile, and have good electric and thermal conductivity. They are usually solid at room temperature, with the exception of mercury, and have a high density.
What does 'malleable' mean in the context of metals?
-Malleable refers to the property of metals that allows them to be hammered into thin sheets, like aluminum or gold.
What are the characteristics of nonmetals as described in the video?
-Nonmetals tend to be brittle, have low elasticity, are poor conductors of heat and electricity, making them good insulators. They are often dull, have low densities, and half of them are gases at room temperature.
What is the difference between metalloids and metals in terms of electrical conductivity?
-Metalloids, also known as semimetals, are electrical semiconductors, meaning they have electrical conductivity between that of metals and nonmetals.
What are alkali metals and where are they located on the periodic table?
-Alkali metals are elements found in Group 1 of the periodic table, excluding hydrogen. They are highly reactive, especially with water, and have one valence electron.
Why are alkali metals never found alone in nature?
-Alkali metals are never found alone in nature because they are highly reactive with water and will react with it if not bonded with another element.
What group on the periodic table contains the alkaline earth metals?
-Alkaline earth metals are found in Group 2 of the periodic table and include elements from beryllium to radium.
What are some physical and chemical properties of the transition metals?
-Transition metals, found in groups 3 through 12, form colored compounds when reacting with other elements. They are less reactive than alkali and alkaline earth metals, have high melting points, and have varying oxidation states.
What does the term 'halogen' mean and which group do they belong to on the periodic table?
-Halogens mean 'salt-producing' and are elements in Group 17 that typically react with metals to form salts. They are highly reactive and have seven valence electrons.
What are noble gases and why are they considered inert?
-Noble gases are elements in Group 18 of the periodic table. They are considered inert or chemically unreactive because they have a completely filled outer energy level.
What are the lanthanides and where can they be found on the periodic table?
-Lanthanides are elements from atomic number 57 to 71 (or 72 depending on the source) and are located in the f-block of the periodic table. They are often referred to as rare earth elements.
What are the actinides and what distinguishes them from other elements?
-Actinides are elements from atomic number 89 to 103 and are found at the bottom of the periodic table. Many actinides are synthetic, man-made elements that do not occur naturally and are often radioactive.
Outlines
🔬 Introduction to Element Classes on the Periodic Table
Mr. Melons introduces the video with an overview of the periodic table's element classes. He explains the basic division of elements into metals, nonmetals, and metalloids based on their position relative to the stair-step line. Metals are typically hard, shiny, and good conductors of heat and electricity, while nonmetals are brittle and poor conductors, making them good insulators. Metalloids have properties of both and are semiconductors. The video will delve into the characteristics of alkali metals, alkaline earth metals, and other groups.
🌌 Exploring the Properties of Alkali and Alkaline Earth Metals
This section discusses alkali metals, found in Group 1 excluding hydrogen, which are highly reactive, especially with water, and have one valence electron. As you move down the group, their reactivity increases. They are never found alone in nature due to their reactivity with water and have low densities, boiling points, and melting points, and are soft enough to cut with a knife. Alkaline earth metals, found in Group 2, have two valence electrons and are also reactive, forming strong bases with water, and share similar properties with alkali metals but differ in their reactivity and appearance.
🌈 Transition Metals, Halogens, and Noble Gases
Transition metals, located in groups 3 through 12, form colored compounds and are less reactive than alkali and alkaline earth metals. They have high melting points and can have varying oxidation states. Halogens, found in Group 17, are known as salt-producing elements and are highly reactive, forming diatomic molecules and never found alone in nature. Noble gases, in Group 18, are chemically inert and stable, typically colorless, odorless, and monatomic, with a completely filled outer energy level.
📚 Lanthanides, Actinides, and Acknowledgments
Lanthanides, elements 57 to 71 on the periodic table, are often referred to as rare earth elements. They are reactive metals with high melting and boiling points. Actinides, from element 89 to 103, are radioactive, tarnish in air, and are often man-made. The video concludes with acknowledgments for the sources of the element pictures used in the video, and an invitation for viewers to engage with the content by liking and commenting.
Mindmap
Keywords
💡Periodic Table
💡Metals
💡Nonmetals
💡Metalloids
💡Alkali Metals
💡Alkaline Earth Metals
💡Transition Metals
💡Halogens
💡Noble Gases
💡Lanthanides
💡Actinides
Highlights
Introduction to different element classes on the periodic table.
Explanation of metals as elements to the left of the stair-step line, with the exception of hydrogen.
Nonmetals are elements to the right of the stair-step line.
Semi-metals or metalloids lie directly on the stair-step line, with exceptions like aluminum.
Physical properties of metals include hardness, shininess, malleability, ductility, and good conductivity.
Malleability allows metals to be hammered into thin sheets.
Metals' ductility enables them to be made into wires.
Most metals are solid at room temperature, except for mercury.
Nonmetals are brittle, poor conductors of heat and electricity, and often good insulators.
Examples of nonmetals include chlorine gas, sulfur, carbon, and phosphorus.
Metalloids or semimetals have properties of both metals and nonmetals and are good semiconductors.
Alkali metals in Group 1 are highly reactive with water and have one valence electron.
Alkaline earth metals in Group 2 have two valence electrons and form strong bases with water.
Transition metals in groups 3 through 12 form colored compounds and have varying oxidation states.
Halogens in Group 17 are highly reactive and form diatomic molecules.
Noble gases in Group 18 are inert and chemically unreactive due to a full outer energy level.
Lanthanides and actinides are often man-made and have unique properties like radioactivity and high reactivity.
Acknowledgment of the sources of images and information used in the video.
Transcripts
Browse More Related Video

Periodic Table of Elements Song

3.2 Introduction to the Periodic Table | High School Chemistry

Periodic Table of Elements Explained - Metals, Nonmetals, Valence Electrons, Charges

The periodic table | Atoms, elements, and the periodic table | High school chemistry | Khan Academy
The Periodic Table of the Elements in Chemistry - [1-2-12]

Periodic Table Explained: Name Origin
5.0 / 5 (0 votes)
Thanks for rating: