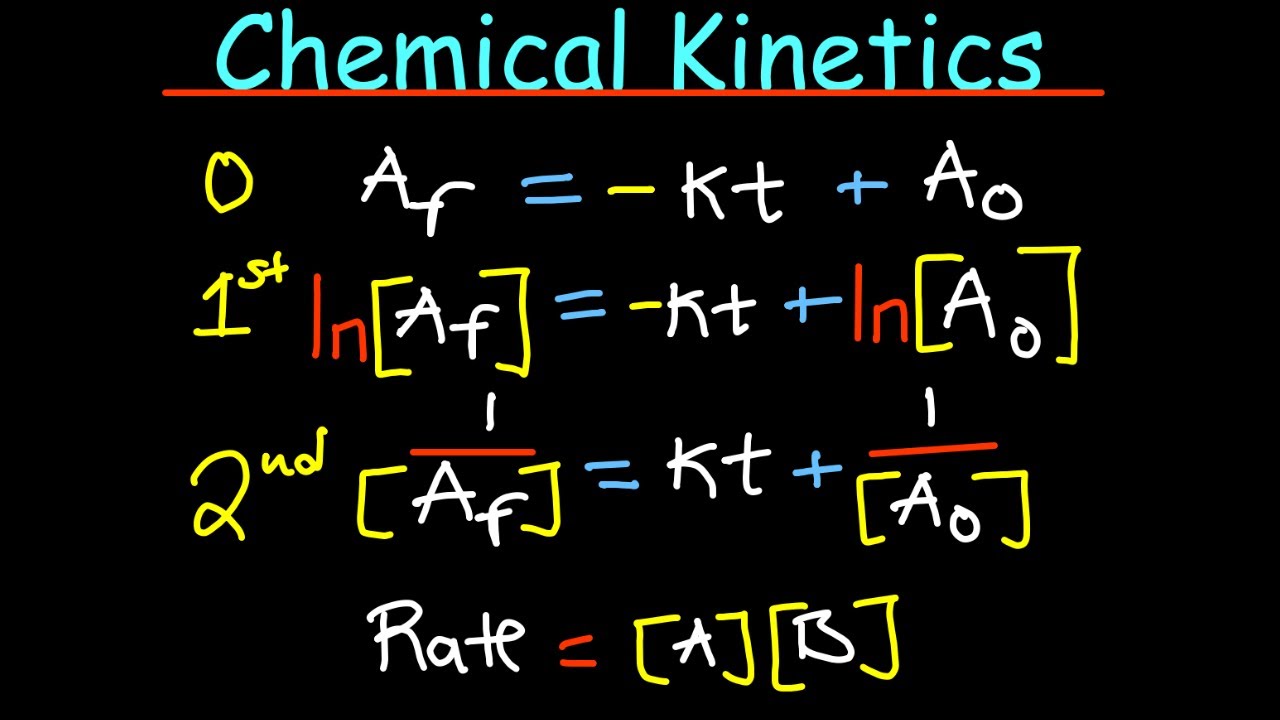
An Introduction to Chemical Kinetics
TLDRThis lecture delves into the realm of chemical kinetics, focusing on the rates of chemical reactions and their implications for understanding reaction mechanisms. The instructor emphasizes that kinetics is not just about speed but also uncovering the processes that enable practical applications like catalyst development and drug creation. Using the example of NO2 and CO reaction, the lecture illustrates the difference between the overall reaction and the detailed mechanism, highlighting factors affecting reaction rates such as physical state, concentration, temperature, and catalysts. The importance of measuring rates to deduce mechanisms is underscored, with practical examples and problems to solidify the concepts.
Takeaways
- 🔬 Chemical kinetics is the study of reaction rates and provides insight into reaction mechanisms, which is crucial for practical applications in chemistry.
- 🧬 The speed of a reaction is not the only important aspect of kinetics; understanding the mechanism is key to manipulating chemical processes effectively.
- 🔍 An example of a reaction mechanism is the conversion of NO2 and CO into NO and CO2, which occurs in two steps, showing that the overall reaction does not always reflect the actual mechanism.
- 🌡 Factors affecting reaction rates include the physical state of reactants, their concentrations, reaction temperature, and the presence of catalysts.
- 📉 The rate of a chemical reaction is defined as the change in concentration of a reactant or product over time, and it can be determined by monitoring one component of the reaction.
- 📈 The rate of a reaction can be represented graphically, with the slope of the curve at any point indicating the instantaneous rate.
- 📊 The instantaneous rate at time zero is often of interest as it provides a starting point for understanding how the rate changes over the course of the reaction.
- ⏱ As a reaction progresses, the rate typically decreases over time due to the decreasing concentration of reactants.
- 🔢 The rate of a reaction can be influenced by stoichiometric coefficients, which must be accounted for when calculating rates from changes in concentration.
- 📚 Understanding the relationship between reactants and products in terms of their rates allows chemists to measure the rate of one to infer the rate of the other.
- 🛠 Kinetics is foundational for developing a deeper understanding of reaction mechanisms, which is essential for the development of catalysts, drugs, and other chemical interventions.
Q & A
What is chemical kinetics and why is it important in chemistry?
-Chemical kinetics is the area of science that studies the rates at which chemical processes occur. It is important because it provides insights into the reaction mechanism, which is key to using chemistry for practical applications such as creating catalysts or inventing drugs.
What is the main difference between the overall reaction and the reaction mechanism?
-The overall reaction describes the initial reactants and final products without detailing the process. The reaction mechanism, on the other hand, explains the step-by-step process at the molecular level, showing how reactants are converted into products.
Can you provide an example of a reaction mechanism from the script?
-The script provides the example of NO2 reacting with CO to form NO and CO2. The mechanism involves two steps: first, two NO2 molecules react to form NO3, and then NO3 reacts with CO to produce NO and CO2.
What are the factors that affect the speed of chemical reactions?
-The factors affecting reaction speeds include the physical state of the reactants, their concentrations, the reaction temperature, and the presence of a catalyst.
Why is the physical state of reactants significant in determining reaction rates?
-The physical state is significant because it influences the frequency of collisions between reactant molecules. Gases typically have higher reaction rates than liquids and solids due to more frequent collisions.
How does concentration affect the rate of a chemical reaction?
-Concentration affects the rate of a reaction because a higher concentration of reactants increases the likelihood of collisions between molecules, leading to a faster reaction rate.
What is the relationship between temperature and reaction rates?
-Higher temperatures generally lead to faster reaction rates because the molecules have more energy and move faster, increasing the frequency and energy of collisions, which can help overcome activation energy barriers.
What is the definition of a reaction rate in terms of chemical kinetics?
-The reaction rate is defined as the change in concentration of a reactant or product over a change in time. It is typically expressed in units of molarity per second or similar.
How can you determine the rate of a reaction experimentally?
-You can determine the rate of a reaction experimentally by monitoring the change in concentration of a reactant or product over time, using techniques such as spectroscopy, and then calculating the rate using the formula for rate of change.
What is the significance of the instantaneous rate in understanding reaction kinetics?
-The instantaneous rate represents the rate of the reaction at a specific moment in time. It is important for understanding the dynamics of the reaction, especially at the beginning (initial rate), and can be visualized graphically as the slope of a tangent to a concentration-time plot.
How does the stoichiometry of a reaction relate to its rate?
-The stoichiometry of a reaction, represented by coefficients in the balanced equation, relates to the rate by indicating how changes in the concentration of reactants and products are proportional to each other. For example, if a reactant A is consumed to produce three units of product B, the rate of disappearance of A will be one-third the rate of appearance of B.
Outlines
🔍 Introduction to Chemical Kinetics and Reaction Mechanisms
The script begins with an introduction to chemical kinetics, emphasizing its importance in understanding not just the speed of chemical reactions but also the underlying mechanisms. It clarifies that while the public often associates kinetics with reaction speed, its true value lies in revealing reaction mechanisms, which is crucial for practical applications like catalyst development and drug design. The lecturer uses the example of the reaction between NO2 and CO to form NO and CO2, illustrating that the reaction mechanism is not always straightforward and requires the study of kinetics to be fully understood.
🌡 Factors Influencing Reaction Rates
This paragraph delves into the factors that affect the rate of chemical reactions. It discusses the physical state of reactants, noting that gases typically react faster than liquids and solids due to increased collision frequency. The paragraph also covers the impact of concentration, temperature, and the presence of a catalyst on reaction rates. Temperature is highlighted for its dual role in increasing collision frequency and energy, potentially overcoming activation energy barriers. The segment concludes with an introduction to the concept of reaction rates as rates of change, defined by the change in concentration over time, and the importance of molar concentration and time in these measurements.
📊 Calculating Reaction Rates and Understanding Rate Dynamics
The script explains how to calculate reaction rates by demonstrating the process using a hypothetical chemical reaction. It describes the method of determining the rate of product formation or reactant disappearance, emphasizing the importance of units and the convention of using a negative sign for reactants to ensure that rate calculations are positive. The paragraph also introduces the concept of average and instantaneous rates, illustrating how these rates can be derived from tabulated data or graphical representations, such as concentration versus time graphs. The significance of the slope of the curve on a graph as an indicator of the instantaneous rate is also discussed.
📚 Analyzing Reaction Rates Over Time and Approaching Equilibrium
This section of the script examines how reaction rates change over time, using a tabulated dataset to demonstrate the calculation of average rates from subsets of data. It explains that as reactants are consumed, the rate of reaction decreases, leading to the concept of equilibrium where forward and reverse reactions occur at the same rate. The paragraph also touches on the practicality of graphing reaction data for easier analysis and understanding, and how the slope of the graph at any point represents the instantaneous rate, with calculus being used to describe these rates more precisely.
📉 Practice Problems and the Significance of Stoichiometric Coefficients
The script presents a practice problem to reinforce the understanding of reaction rates, stoichiometry, and their relationship. It explains how to order rates from fastest to slowest based on the concept that reaction rates decrease over time. The paragraph further explores how stoichiometric coefficients in a balanced chemical equation affect the calculation of rates, using the example of ozone decomposing into oxygen to illustrate the division of rate by the coefficient of the reactant when calculating the rate of product formation.
🔄 Relating Rates of Reactants and Products in Chemical Reactions
This paragraph focuses on the relationship between the rates of reactants and products in a chemical reaction, given their stoichiometric coefficients. It provides a step-by-step guide on how to calculate the rate of change of one substance based on the known rate of change of another, using a hypothetical reaction as an example. The explanation includes the cancellation of negative signs and the application of stoichiometric ratios to find the rate of product formation from the rate of reactant disappearance. The importance of understanding these relationships for future studies in kinetics, including rate laws and reaction mechanisms, is highlighted.
🚀 Conclusion and Preview of Future Kinetics Topics
In conclusion, the script wraps up the lecture by summarizing the key points covered, including the calculation of reaction rates, the impact of stoichiometry, and the graphical representation of reaction data. It also previews upcoming topics in the series of lectures on kinetics, such as rate laws, the distinction between first and second-order reactions, and the study of catalysis and enzymes, setting the stage for a deeper dive into understanding reaction mechanisms.
Mindmap
Keywords
💡Kinetics
💡Reaction Mechanism
💡Chemical Reaction
💡Rate of Reaction
💡Concentration
💡Temperature
💡Catalyst
💡Activation Energy
💡Instantaneous Rate
💡Stoichiometry
💡Equilibrium
Highlights
Chemical kinetics is the study of the rates at which chemical processes occur and its importance in understanding reaction mechanisms.
Kinetics is crucial for practical applications such as creating catalysts and inventing drugs that affect chemical processes in the body.
The example of NO2 and CO reaction demonstrates the difference between the overall reaction and the actual reaction mechanism involving intermediate steps.
Chemical kinetics uses rate measurements to deduce the underlying mechanisms of reactions, which is key to understanding complex chemistry.
Factors affecting reaction rates include the physical state, concentration, temperature, and the presence of a catalyst.
Reaction rates are calculated as the change in concentration over time, a fundamental concept applied to chemistry.
The rate of a reaction can be determined by monitoring either the formation of a product or the disappearance of a reactant.
The rate of a reaction is typically represented as positive, even when considering the disappearance of a reactant.
Graphical representation of reaction rates over time can provide insights into the instantaneous rate at any given point.
The slope of the concentration versus time graph at any point represents the instantaneous rate of the reaction.
The initial rate of a reaction is of particular interest as it provides insights into the reaction's onset without long observation periods.
As reactions progress, the rate decreases over time due to the consumption of reactants and the consequent reduction in collision opportunities.
Stoichiometric coefficients in a balanced chemical equation affect how the rates of reactants and products are related.
Practice problems illustrate the application of stoichiometry in calculating rates from changes in reactant and product concentrations.
Understanding the relationship between stoichiometric coefficients and reaction rates is essential for accurately measuring reaction kinetics.
The lecture concludes with a look forward to future lessons on rate laws, order of reactions, and their implications for understanding reaction mechanisms.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: