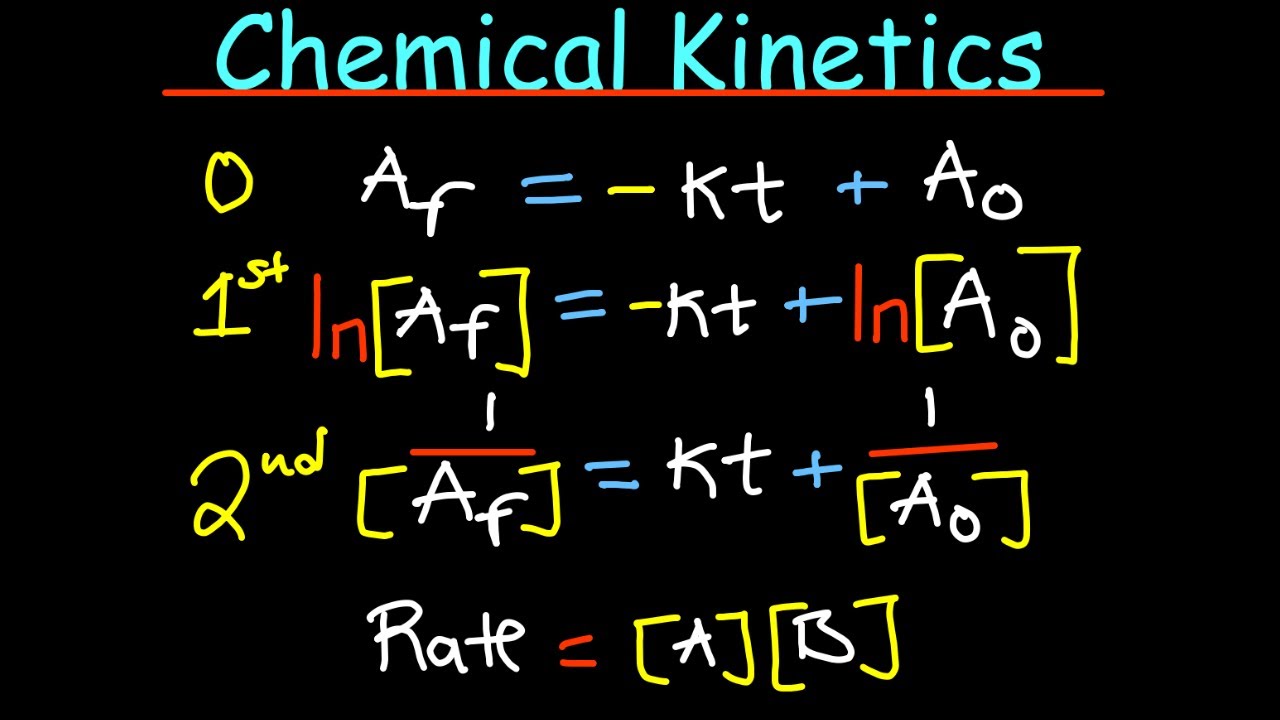
Reaction Rates & Introduction to Kinetics - AP Chem Unit 5, Topic 1
TLDRThe video script provides an insightful introduction to chemical kinetics, focusing on the rates of chemical reactions and the factors that influence these rates. It begins with an exploration of how different chemical reactions can vary greatly in speed, from explosively fast to slow rusting processes. The script then delves into the definitions and methods of measuring reaction rates, using a balanced chemical equation as an example to illustrate how the coefficients in the equation can indicate relative rates of reactants and products. The importance of graphing concentration over time is highlighted, showing how the rate of reaction typically decreases as the reaction progresses. The script also explains how to calculate the rate of change for a substance during a specific time interval and introduces the concept of instantaneous rate. Furthermore, it discusses four primary ways to accelerate a chemical reaction: increasing temperature, raising concentration or pressure, decreasing particle size, and adding a catalyst. Each method is explained with a focus on its molecular impact, such as increasing molecular movement and collisions or providing a lower energy pathway for the reaction. The summary concludes with an encouragement to continue learning about chemical kinetics, fostering an interest in the subject.
Takeaways
- 🔍 Chemical kinetics is the study of the rates of chemical reactions and the factors that affect these rates.
- 🔄 The rate of a reaction can be represented by the change in concentration of reactants or products over time.
- ⚖️ In a balanced chemical equation, substances with the same coefficient have the same rate of reaction.
- 📈 The rate of a reaction is highest at the beginning and decreases as the reaction proceeds.
- 📉 The slope of the concentration vs. time graph represents the rate of reaction, with the steepest slope at the start.
- 🧮 The rate of reaction can be calculated using the change in concentration over a specific time interval.
- 🔩 To find the instantaneous rate at a specific time, one would calculate the slope of the tangent line to the concentration vs. time curve at that point.
- 🔄 The mole ratio from the balanced equation can be used to determine the relative rates of reaction for different substances.
- ⏫ Four ways to speed up a chemical reaction include increasing temperature, increasing concentration, decreasing particle size, and adding a catalyst.
- 🌡️ Raising the temperature increases the rate of reaction by causing molecules to move faster and collide more frequently and with greater force.
- 🛠️ Decreasing particle size increases the surface area, providing more active sites for the reaction to occur and thus speeding up the reaction.
- 💡 Catalysts speed up reactions by providing a lower energy pathway, allowing more molecules to attain the energy threshold necessary for the reaction to occur.
Q & A
What is the primary focus of Unit 5 in the series of units discussing chemical reactions?
-Unit 5 focuses on chemical kinetics, specifically studying the rates of chemical reactions and the factors that affect those rates.
How does the rate of a chemical reaction relate to the coefficients in a balanced chemical equation?
-In a balanced chemical equation, substances with the same coefficients will have the same rate of reaction. If it's a product, its concentration increases at that rate, and if it's a reactant, its concentration decreases at that rate.
What is the mathematical approach to determine the rate of change of a substance in a chemical reaction over a specific time interval?
-The rate of change is calculated by taking the change in concentration of the substance divided by the change in time, expressed as concentration unit per time unit (e.g., molarity per second).
How can you estimate the instantaneous rate of a reaction at a specific moment in time?
-To estimate the instantaneous rate, you would calculate the slope of the tangent line to the concentration vs. time curve at that specific moment. The absolute value of this slope represents the instantaneous rate.
What is the general rule of thumb regarding the effect of temperature on the rate of a typical chemical reaction?
-For every 10°C increase in temperature, the rate of a typical reaction approximately doubles.
How does increasing the concentration of reactants affect the rate of a chemical reaction?
-Increasing the concentration of reactants leads to more frequent collisions between molecules, which in turn increases the likelihood of a successful reaction and thus speeds up the reaction rate.
What is the effect of decreasing particle size on the rate of a chemical reaction?
-Decreasing particle size increases the surface area of the reactants, providing more active sites for the reaction to occur, which speeds up the reaction rate.
How does a catalyst influence the rate of a chemical reaction?
-A catalyst speeds up a reaction by providing a lower energy alternate pathway, allowing more molecules to attain the activation energy and react, without being consumed in the process.
Why are all rates of reaction in chemistry considered positive, regardless of whether the concentration of a substance is increasing or decreasing?
-In chemistry, by convention, all rates are considered positive. This is similar to a car's speedometer, where reverse is still a positive speed. It simplifies the concept and avoids confusion about the direction of change.
What are the four main factors that can be manipulated to speed up a chemical reaction?
-The four main factors to speed up a chemical reaction are raising the temperature, increasing the concentration (or pressure for gases), decreasing the particle size, and adding a catalyst.
How does the rusting of a car serve as an example of a slow chemical reaction in the script?
-The rusting of a car is used as an example of a slow chemical reaction to contrast with the fast reactions, like combustion or explosions. It illustrates that chemical kinetics encompasses a wide range of reaction speeds.
Outlines
🔍 Introduction to Chemical Kinetics
This paragraph introduces the topic of chemical kinetics, which is the study of reaction rates and the factors influencing them. It sets the stage for a series of units that explore different aspects of chemical reactions, such as thermodynamics and equilibrium. The paragraph uses examples of fast and slow reactions to illustrate the importance of understanding reaction rates. It also introduces the concept of measuring reaction rates through changes in concentration over time, using a balanced chemical equation and a graph to demonstrate how reactants and products' concentrations change during a reaction.
📈 Understanding Reaction Rates Over Time
The second paragraph delves into how the rate of a chemical reaction changes over time. It explains that the rate of reaction is highest at the beginning and decreases as the reaction progresses. The paragraph provides a mathematical approach to determining the rate of change of a substance, such as nitrogen dioxide (N2O), by calculating the slope of the concentration-time graph. It also discusses the concept of instantaneous rate, which is the rate at a specific moment in time, and how it can be found by taking the slope of a tangent line to the curve at that point.
🔄 Using Coefficients to Determine Relative Reaction Rates
This paragraph explains how to use the coefficients from a balanced chemical equation to estimate the relative rates of reaction for different substances. It demonstrates that substances with the same coefficient have the same rate of reaction, with products increasing and reactants decreasing at the same rate. The paragraph also covers how to calculate the rate of change for other substances involved in the reaction by using the mole ratio, as illustrated with the example of oxygen in relation to N2O.
⏫ Ways to Accelerate Chemical Reactions
The final paragraph discusses four methods to speed up chemical reactions, which are also applicable in reverse to slow down reactions. These methods include increasing the temperature, which causes molecules to move faster and collide more frequently and with greater force; increasing the concentration or pressure, which brings reacting molecules closer together; decreasing the particle size, which increases the surface area and the number of active sites for reaction; and adding a catalyst, which provides a lower energy pathway for the reaction, allowing more molecules to participate and thus increasing the reaction rate.
Mindmap
Keywords
💡Chemical Kinetics
💡Reaction Rates
💡Balanced Equation
💡Concentration
💡Temperature
💡Particle Size
💡Catalyst
💡Activation Energy
💡Stoichiometry
💡Equilibrium
💡Thermodynamics
Highlights
Unit 5 introduces the fundamentals of chemical kinetics, focusing on the rates of chemical reactions and factors affecting those rates.
Chemical kinetics answers the question of how fast a reaction will proceed, contrasting with thermodynamics and equilibrium which address if and how far a reaction will occur.
The rate of a chemical reaction can be visually represented through a graph plotting concentration against time.
In a balanced chemical equation, substances with the same coefficient have the same rate of reaction, whether increasing as a product or decreasing as a reactant.
The rate of reaction typically starts high and decreases over time, observable through the slope of the concentration-time graph.
The rate of change of a substance in a reaction is calculated as the change in concentration over the change in time.
Instantaneous rate at a specific moment can be determined by the slope of a tangent line to the concentration-time curve at that point.
The mole ratio from the balanced equation can be used to estimate the relative rates of reaction for different substances.
In chemistry, rates of reaction are conventionally expressed as positive values, regardless of the direction of change.
Four ways to speed up a chemical reaction include changing the temperature, increasing the concentration, decreasing the particle size, and adding a catalyst.
Raising the temperature increases the rate of reaction by causing molecules to move faster and collide more frequently and with greater force.
Increasing the concentration or pressure leads to more frequent collisions between molecules, potentially speeding up the reaction.
Decreasing the particle size of a reactant increases its surface area, providing more active sites for the reaction to occur.
A catalyst speeds up a reaction by providing a lower energy pathway, allowing more molecules to attain the energy threshold for reaction.
Catalysts are particularly useful as they can be recycled and do not get consumed in the reaction process.
Understanding these factors is crucial for controlling reaction rates in both laboratory and industrial settings.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: