Chemical Kinetics Full Review
TLDRThe video script delves into the intricacies of chemical kinetics, focusing on the study of reaction rates and mechanisms. It introduces the concept of reaction rates as the change in concentration of reactants and products over time, and explores factors affecting these rates, such as temperature, concentration, surface area, and pressure. The script also explains the Collision Theory and its significance in understanding how chemical reactions progress. Detailed examples and explanations are provided to illustrate how to determine the rate of a chemical reaction, including the use of stoichiometric coefficients and the calculation of instantaneous and average rates. The importance of understanding rate laws and the order of reactions is emphasized, with practical applications of these concepts in mind.
Takeaways
- 📚 Chemical kinetics is the study of reaction rates and reaction mechanisms, focusing on how changes in concentration of reactants and products occur over time.
- 🔍 The rate of a chemical reaction can be expressed as the change in concentration of a reactant or product per unit time, often denoted by small letters and stoichiometric coefficients.
- 🎢 Understanding reaction mechanisms involves examining the sequence of events that occur during a reaction, such as collision theory and activation energy concepts.
- 🌡️ Temperature is a key factor affecting reaction rates, as it relates to the average kinetic energy of particles; higher temperatures often lead to increased reaction rates.
- 💧 Concentration of reactants is directly related to the rate of reaction, with higher concentrations leading to more frequent collisions and thus faster reaction rates.
- 📈 The rate law is a mathematical expression that describes the relationship between the rate of a chemical reaction and the concentration of its reactants.
- 🔄 Integrated rate laws are derived from differential rate laws and provide a way to calculate the concentration of reactants at different times during a reaction.
- 🕒 Half-life is the time required for the concentration of a reactant to decrease to half its initial value, a concept particularly relevant for first-order reactions.
- 📊 Graphs of reaction rates can help visualize the behavior of reactions and determine reaction orders, with different graphs for zero-order, first-order, and second-order reactions.
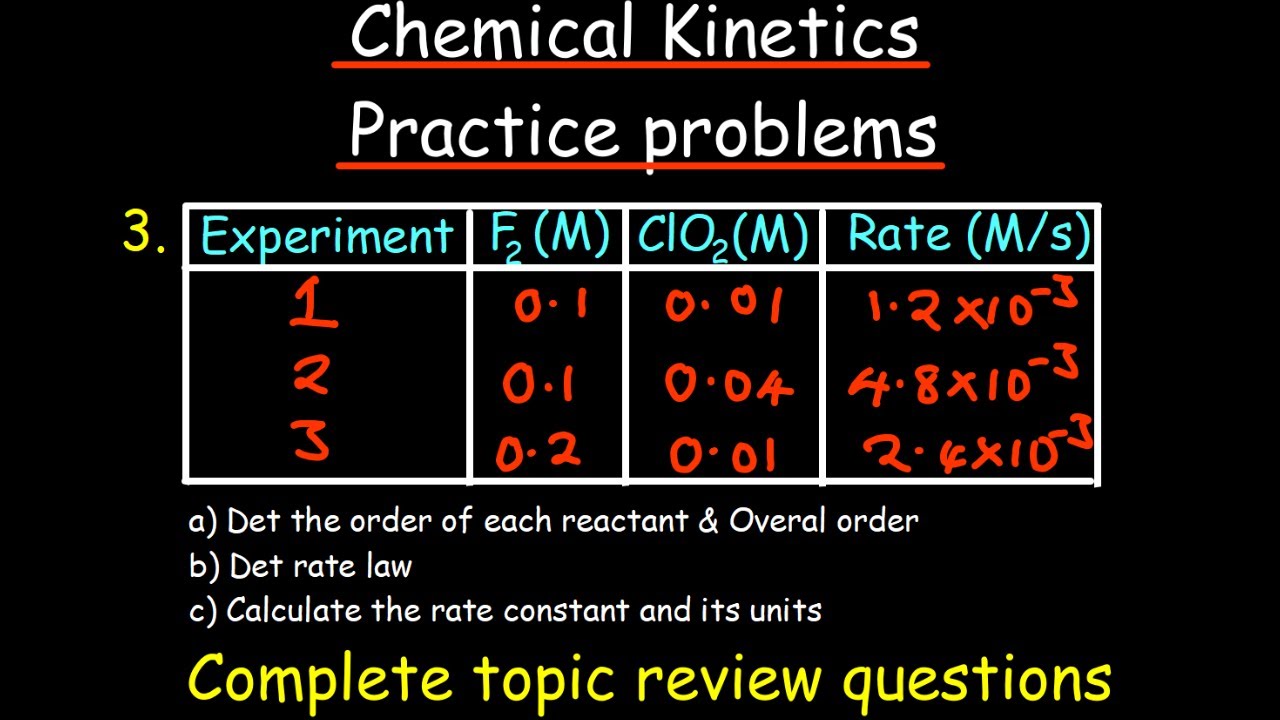
- 🧪 Experimental data is crucial for determining rate constants and reaction orders, often through the method of initial rates and the analysis of how reaction rates change with varying concentrations.
- 🔍 The order of a reaction, whether zero, first, or second, dictates the integrated rate law and helps predict how the concentration of reactants will change over time in a reaction.
Q & A
What is chemical kinetics and what does it study?
-Chemical kinetics is the study of reaction rates and reaction mechanisms. It involves examining the changes in concentrations of reactants and products over time and understanding the underlying processes that allow a reaction to occur, such as collision theory and activation energy concepts.
How is the rate of a chemical reaction defined?
-The rate of a chemical reaction is defined as the change in concentration of reactants or products per unit time. It can be expressed as the rate of change of concentration or concentrations of reactants and products with respect to time.
What are the factors that affect the rate of a chemical reaction?
-Factors affecting the rate of a chemical reaction include temperature, concentration of reactants, surface area of solids, and pressure for gases. These factors influence the likelihood and frequency of successful collisions between reactant particles, which in turn affect the reaction rate.
How does temperature affect the rate of a chemical reaction?
-An increase in temperature leads to an increase in the kinetic energy of the particles, causing them to move faster. This results in a higher probability of successful collisions with sufficient energy, thereby increasing the rate of the chemical reaction.
What is the role of activation energy in chemical kinetics?
-Activation energy is the minimum amount of energy required for a chemical reaction to proceed. It is a threshold that reactant particles must overcome through collision to successfully react and form products.
What are the different types of rates in chemical kinetics?
-The different types of rates in chemical kinetics include instantaneous rate, which is the rate at a specific moment in time, and average rate, which is the overall rate observed over a period of time.
How can you determine the rate law for a given chemical reaction?
-The rate law for a chemical reaction is determined by experimental data, where initial rates are measured for different concentrations of reactants. By analyzing how changes in concentration affect the rate, one can establish the relationship between the rate of reaction and the concentrations of the reactants, expressed as the rate law.
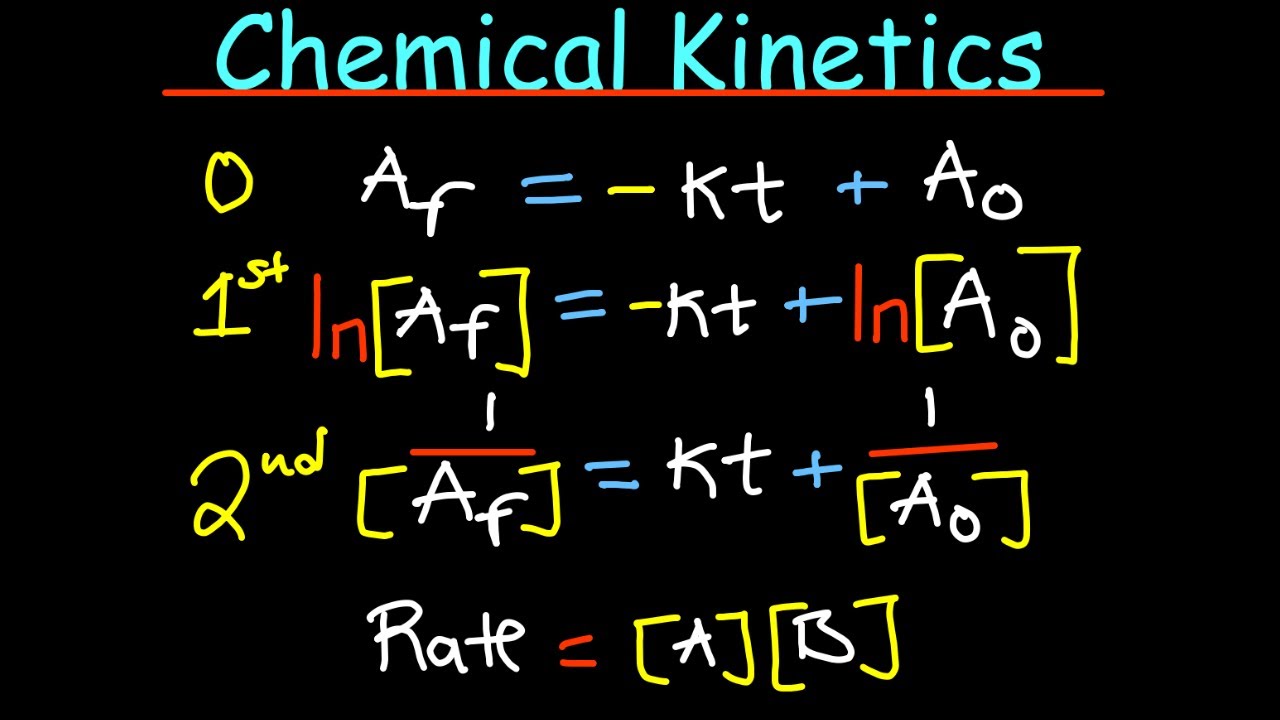
What is the integrated rate law for a zero-order reaction?
-The integrated rate law for a zero-order reaction is expressed as the final concentration being equal to the initial concentration minus the product of the rate constant (k) and the time (t).
How does the concept of half-life relate to first-order reactions?
-In first-order reactions, the half-life is the time required for the concentration of a reactant to decrease to half of its initial value. It is a constant for a given reaction and is independent of the initial concentration of the reactant.
What is the relationship between the rate constant (k) and the rate law in chemical kinetics?
-The rate constant (k) in the rate law represents the proportionality between the rate of the chemical reaction and the concentrations of the reactants raised to their respective reaction orders. It is a value that is specific to a given reaction and is influenced by factors such as temperature.
How can you determine the order of a reactant in a chemical reaction?
-The order of a reactant in a chemical reaction can be determined by observing how changes in the concentration of that reactant affect the rate of the reaction. If the rate changes by a factor of 'n' when the concentration is doubled, then the reactant is of the first order. If the rate does not change with the concentration of the reactant, it is of zero order.
Outlines
📚 Introduction to Chemical Kinetics
The paragraph introduces the topic of chemical kinetics, which is the study of reaction rates and mechanisms. It emphasizes the importance of understanding how changes in concentration of reactants and products occur over time. The speaker expresses excitement about covering the topic in detail and mentions the use of Collision Theory to explain reaction mechanisms. The introduction sets the stage for a comprehensive discussion on the factors affecting reaction rates and the mathematical expressions used to describe these rates.
🧪 Example of Reaction Rates
This paragraph provides a simple example to illustrate the concept of reaction rates. It explains how to calculate the rate of a chemical reaction by using the change in concentration of reactants or products over a specific time period. The example uses a hypothetical reaction between substances A and B to produce C and D, and it demonstrates how stoichiometric coefficients are used in the rate equation. The explanation includes a discussion on why negative values are used for reactants and positive values for products, clarifying that the result of the rate calculation will always be a positive value.
🌡️ Factors Affecting Reaction Rates
The paragraph delves into the factors that influence the rate of a chemical reaction. It focuses on the Collision Theory, which posits that reactant particles must collide with sufficient energy for a reaction to occur. The speaker explains how temperature and concentration of reactants affect the rate, with higher temperatures and concentrations leading to more frequent collisions and thus faster reactions. The paragraph also touches on the concept of activation energy and how it relates to the energy needed for successful collisions between reactant particles.
🔧 Reaction Rate Determination
This section discusses how to determine the rate of a chemical reaction, particularly in the context of a decomposition reaction. It explains the difference between average rate, instantaneous rate, and initial rate, providing examples of how to calculate each. The speaker clarifies that the average rate considers the total time span of the reaction, while the instantaneous rate refers to the rate at a specific point in time. The paragraph also highlights the importance of understanding the stoichiometric coefficients when calculating reaction rates.
📈 Understanding Rate Laws
The paragraph introduces rate laws, which are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its reactants. It explains that the rate law is dependent on a constant (k) and the concentration of reactants raised to certain powers (the orders of the reaction). The speaker provides an example of a reaction between nitrogen dioxide and carbon monoxide, explaining how to determine the rate law for this specific reaction based on given orders of reactants.
🔍 Determining Reaction Orders
This section focuses on how to determine the reaction orders for a given chemical reaction using experimental data. It explains the process of comparing initial rates and concentrations of reactants across different experiments to deduce the order of the reaction with respect to each reactant. The speaker uses a hypothetical reaction between substances A and B to illustrate the method, showing how changes in concentration lead to changes in reaction rate and how this information can be used to calculate the reaction orders (X and Y).
📊 Integrated Rate Laws and Half-Life
The paragraph discusses integrated rate laws, which are derived from integrating the differential rate laws. It provides equations for zero-order, first-order, and second-order reactions, explaining how they relate to the concentration of reactants over time. The concept of half-life is introduced, defining it as the time required for the concentration of a reactant to reduce to half its initial value. The speaker emphasizes the importance of understanding these integrated rate laws and half-life in the study of chemical kinetics, and provides practice questions to reinforce the concepts.
Mindmap
Keywords
💡Chemical Kinetics
💡Reaction Rates
💡Stoichiometric Coefficients
💡Collision Theory
💡Activation Energy
💡Temperature
💡Concentration
💡Surface Area
💡Pressure
💡Rate Law
💡Integrated Rate Laws
Highlights
Chemical kinetics is the study of reaction rates and mechanisms.
Reaction rates can be defined as the rate of change of concentration of reactants and products with respect to time.
Reaction mechanisms involve understanding the steps that occur within a chemical reaction, such as collision theory.
The rate of a chemical reaction can be determined using the formula involving stoichiometric coefficients and concentration changes.
For reactants, the rate formula involves a negative change in concentration, while for products, it is positive.
Factors affecting the rate of a chemical reaction include temperature, concentration, surface area, and pressure.
An increase in temperature leads to an increase in the kinetic energy of particles, resulting in more frequent collisions.
Higher concentration of reactants increases the likelihood of particle collisions, thus increasing the reaction rate.
The rate law is a mathematical expression describing the relationship between the rate of a chemical reaction and the concentration of its reactants.
The order of a reaction with respect to a reactant is indicated by the power to which the reactant's concentration is raised in the rate law.
The overall order of a reaction is the sum of the orders of each reactant.
Integrated rate laws are derived by integrating the differential rate laws.
Zero order reactions have a rate independent of the reactant concentration and are integrated to give a straight line.
First order reactions have their integrated rate law involving natural logarithms of concentration.
Second order reactions have integrated rate laws with reciprocals of concentration and a positive gradient.
Half-life is the time taken for the concentration or amount of a reactant to reduce to half its initial value.
The rate constant can be determined using the integrated rate laws and experimental data.
Graphs of reaction orders show different slopes and intercepts, which can be used to identify the order of a reaction.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: