Heat of Fusion and Heat of Vaporization Explained
TLDRIn this educational video, Mr. Millington explains the concepts of latent heat of fusion and latent heat of vaporization, focusing on their applications to phase changes in substances. He illustrates these concepts with examples involving ice melting, water boiling, and condensation on a mirror. The video delves into the specific heats of fusion and vaporization for water, detailing the amount of energy required to change water's state from solid to liquid and from liquid to gas. Mr. Millington also compares these values to those of other substances and provides calculations to demonstrate how to apply these concepts to various scenarios, aiming to help viewers understand and solve thermochemistry problems.
Takeaways
- 🧊 The concept of latent heat of fusion is the amount of energy required to change a solid into a liquid without a change in temperature, such as ice melting into water.
- 💧 The latent heat of vaporization is the energy needed for a liquid to turn into a gas, or vice versa, also without a change in temperature, like water boiling into vapor.
- 🔥 When substances change states, they either absorb (endothermic process) or release (exothermic process) energy, which is quantified by the latent heat of fusion or vaporization.
- 🌡️ The melting point of ice and the boiling point of water are both at 0°C and 100°C respectively, which are critical temperatures for the phase changes discussed.
- 📏 The heat of fusion for water is 334 joules per gram, meaning it takes this amount of energy to convert one gram of ice at 0°C into water.
- 🌀 Conversely, it requires the release of 334 joules per gram for water to freeze into ice, which is an exothermic process.
- 🌫️ For water to turn into vapor at its boiling point of 100°C, it needs to absorb 2260 joules per gram, indicating a significant amount of energy is involved in this endothermic process.
- 🌬️ When water vapor condenses back into liquid water, it releases energy, specifically 2260 joules per gram, which is the heat of vaporization for water.
- 📊 Different substances have different specific heat of fusion and heat of vaporization values, which are unique to their molecular structures and properties.
- 🔢 To calculate energy changes for phase transitions, one must use the specific heat of fusion or heat of vaporization for the substance in question, multiplied by the mass of the substance involved.
- 🔄 The script provides examples to illustrate the calculation of energy associated with changing water and ice into vapor and vice versa, emphasizing the use of correct signs for endothermic and exothermic processes.
Q & A
What is the concept of latent heat of fusion?
-The latent heat of fusion is the amount of energy it takes to turn a solid into a liquid or a liquid back into a solid without changing the temperature.
Can you give an example of a process involving latent heat of fusion?
-An example of a process involving latent heat of fusion is when an ice cube melts into water on a hot summer day, which requires the absorption of energy without a change in temperature.
What is the latent heat of vaporization?
-The latent heat of vaporization is the amount of energy associated with a liquid turning into a gas or a gas turning back into a liquid.
How does the energy change when water boils on a stove?
-When water boils on a stove, it absorbs thermal energy from the stove, which is used to change the state of water from liquid to gas at 100 degrees Celsius.
What is the relationship between the heat of fusion and heat of vaporization for water?
-The heat of fusion for water is the energy required to change ice to water, and the heat of vaporization is the energy needed to change water to water vapor. Both are specific amounts for water and are different processes.
What is the heat of fusion for water in joules per gram?
-The heat of fusion for water is 334 joules per gram, which is the energy required to melt one gram of ice into water.
What is the heat of vaporization for water in joules per gram?
-The heat of vaporization for water is 2260 joules per gram, which is the energy needed to convert one gram of water into water vapor at 100 degrees Celsius.
How does the energy required to boil water compare to the energy required to melt ice, assuming the same mass?
-It takes about seven times more energy to boil water (turn it into vapor) than it does to melt ice (turn it into water), given the same mass and under the same pressure conditions.
How much energy is required to change 100 grams of water to water vapor at 100 degrees Celsius?
-To change 100 grams of water to water vapor at 100 degrees Celsius, 226,000 joules of energy are required.
How much energy is released when 250 grams of water at zero degrees Celsius is turned into ice?
-When 250 grams of water at zero degrees Celsius is turned into ice, 83,500 joules of energy are released.
What is the energy change when 10 grams of ice at zero degrees Celsius is melted into water?
-When 10 grams of ice at zero degrees Celsius is melted into water, 3340 joules of energy must be absorbed.
How much energy is released when 200 grams of water vapor is condensed into water?
-When 200 grams of water vapor is condensed into water, 452,000 joules of energy are released.
Outlines
🧊 Introduction to Latent Heat of Fusion and Vaporization
Mr. Millington introduces the concepts of latent heat of fusion and latent heat of vaporization, explaining that these are the amounts of energy absorbed or released when substances change states without a change in temperature. He uses examples such as an ice cube melting in the sun and water freezing in a freezer to illustrate heat of fusion, and boiling water on a stove and condensation on a mirror to demonstrate heat of vaporization. The video aims to apply these concepts to various examples to clarify the processes and the energy changes involved.
🌡️ Heat of Vaporization and Fusion Specific to Water
The script delves into the specifics of heat of vaporization and fusion for water. It explains that each substance has unique values for these properties, but the focus is on water. The heat of fusion for water is given as 334 joules per gram, which is the energy required to convert ice at 0°C to water without a temperature change. Conversely, the heat of vaporization is 2260 joules per gram, the energy needed to turn water at 100°C into water vapor. The script contrasts these values to emphasize that boiling water requires about seven times more energy than melting ice, assuming equal masses and pressures.
🔢 Calculating Energy Changes in Phase Transitions
This section of the script involves calculations related to phase transitions of water. It provides examples of how to determine the energy required to convert water to vapor and vice versa. For instance, to convert 100 grams of water to vapor at 100°C, the script calculates the energy needed as 226,000 joules, using the heat of vaporization. Similarly, it shows that 250 grams of water releasing energy to become ice would result in the release of 83,500 joules. The calculations are based on the principles of thermochemistry and are intended to help viewers understand the energy dynamics in phase changes.
📚 Summary of Heat of Vaporization and Fusion Concepts
The final paragraph summarizes the concepts discussed in the video and encourages viewers to apply these concepts to solve chemistry problems related to thermochemistry. It reiterates the importance of understanding the energy changes associated with phase transitions, specifically focusing on the heat of fusion and heat of vaporization. The script concludes by hoping that the examples provided help viewers grasp these concepts and solve their own problems, emphasizing the practical application of the theory in various scenarios.
Mindmap
Keywords
💡Latent Heat of Fusion
💡Latent Heat of Vaporization
💡Heat of Fusion
💡Heat of Vaporization
💡Endothermic Process
💡Exothermic Process
💡Phase Change
💡Thermal Energy
💡Boiling Point
💡Freezing Point
💡Joules
Highlights
Introduction to the concepts of latent heat of fusion and latent heat of vaporization.
Explanation of latent heat of fusion as the energy required to change a solid to a liquid or vice versa.
Example of an ice cube melting on a hot summer day to illustrate heat of fusion.
Description of how water freezes in a freezer, relating to heat of fusion.
Introduction to latent heat of vaporization and its relation to liquid-gas phase changes.
Example of boiling water on a stove to demonstrate heat of vaporization.
Explanation of condensation as an exothermic process using the example of a cold mirror fogging up.
Differentiation between the energy required for diffusion and phase change processes.
Specific values for the heat of fusion and heat of vaporization of water.
Process of ice melting at 0°C and the associated energy absorption.
Process of water freezing and the associated energy release.
Calculation of energy required to convert water to water vapor at its boiling point.
Comparison of energy needed for water vaporization versus ice melting for the same mass.
Table of heat of fusion and heat of vaporization for various substances.
Example calculation of energy for converting 100 grams of water to water vapor.
Example calculation of energy associated with changing 250 grams of water to ice.
Example calculation of energy required to melt 10 grams of ice into water.
Example calculation of energy released when 200 grams of water vapor condense into water.
Summary of the importance of understanding heat of vaporization and heat of fusion in thermochemistry.
Transcripts
Browse More Related Video

Latent Heat of Fusion and Vaporization, Specific Heat Capacity & Calorimetry - Physics

Specific heat, heat of fusion and vaporization example | Chemistry | Khan Academy

Heats of Fusion & Vaporization

Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams
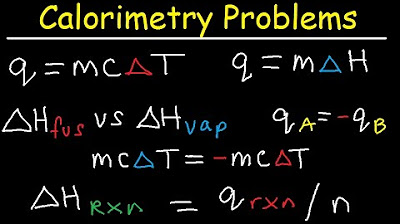
Calorimetry Problems, Thermochemistry Practice, Specific Heat Capacity, Enthalpy Fusion, Chemistry

Phase Changes | Chemistry | The Good and the Beautiful
5.0 / 5 (0 votes)
Thanks for rating: