The Origin of Quantum Mechanics (feat. Neil Turok)
TLDRThe origin of quantum theory is rooted in the quest for more efficient light bulbs in the 1890s. Max Planck, tasked with this challenge, faced discrepancies between his predictions based on electromagnetic theory and experimental results. In a pivotal 'act of despair,' he abandoned the existing theory and derived a new rule: light waves carry energy in discrete packets or 'quanta,' with high-frequency light having larger energy packets and low-frequency light, smaller ones. This concept was initially considered radical but was later related to familiar scenarios by Einstein, such as sharing a single cookie among an increasing number of children, illustrating the quantized nature of energy distribution. Planck's theory revealed that high-frequency light waves, behaving like picky children demanding specific cookie amounts, carry energy in large packets, thus preventing an infinite absorption of energy. This insight led to the understanding that temperature is related to the average energy carried by these quanta. Consequently, as objects heat up, they emit light across the spectrum, from infrared to ultraviolet, a phenomenon observable in the changing colors of a heated object. Planck's quantum theory not only revolutionized light bulb technology by suggesting a filament temperature of around 3200 Kelvin for optimal visible light emission but also hinted at the underlying quantum physics that has been a part of human experience since the dawn of fire-making.
Takeaways
- 💡 Quantum theory originated from a practical problem: increasing the efficiency of light bulbs.
- 🔍 Max Planck was tasked with predicting the light output of a hot filament, which led to the birth of quantum theory.
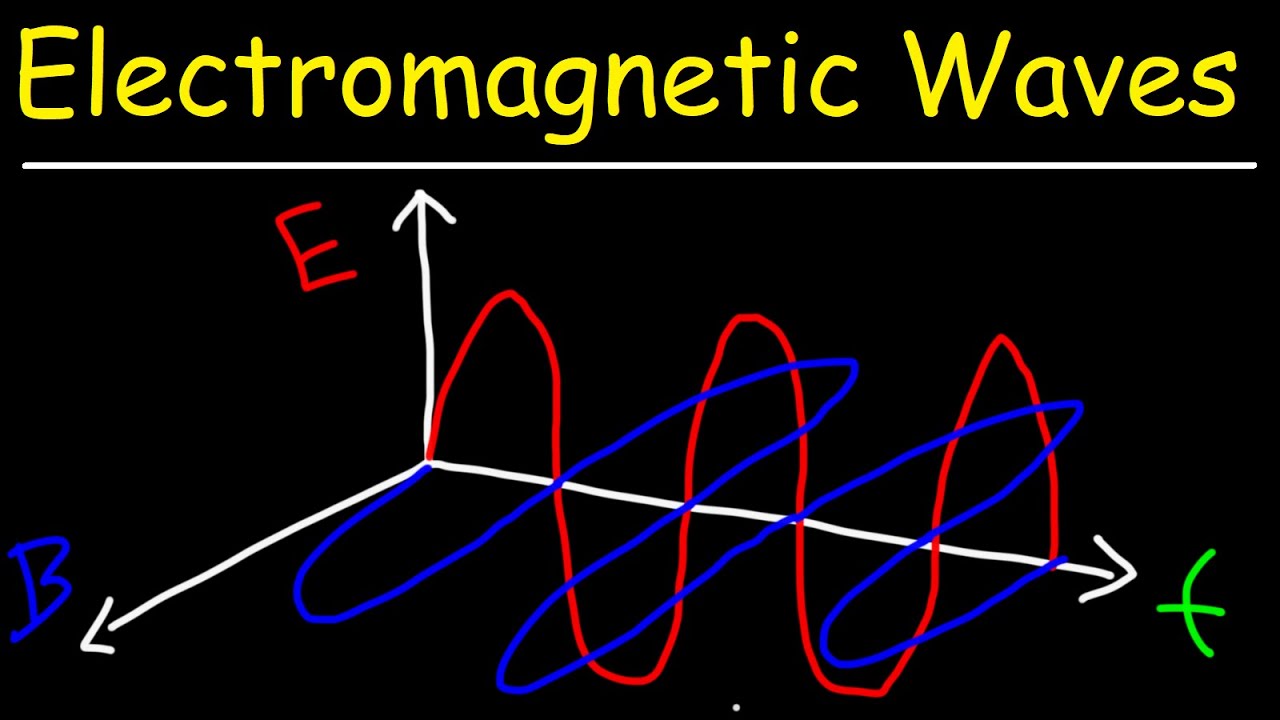
- 🌈 Light is made up of electromagnetic waves with different frequencies corresponding to different colors.
- 🚫 Planck's initial predictions using electromagnetic theory did not align with experimental results.
- 🔁 In a desperate move, Planck discarded the existing theory and derived a new rule from experimental data.
- ⚡ Light waves carry energy in discrete packets, with high frequency light having larger packets and low frequency light having smaller ones.
- 🍪 Einstein related the quantum idea to the problem of sharing, illustrating how energy distribution works with quanta.
- ♾️ The concept of infinitesimally small light waves allows for an infinite number of them to exist in a confined space.
- 🧊 Without quantization, light waves of all sizes could theoretically absorb all available energy, causing a rapid freeze.
- 🎁 High frequency light waves are 'fussy' and only carry energy in specific, large packets, preventing energy from being drained infinitely.
- 🌡️ Temperature is defined by the average energy carried by these packets, with higher temperatures corresponding to higher average energy and higher frequencies of light emitted.
- 💡 According to Planck's quantum theory, light bulb filaments should be heated to about 3200 Kelvin for optimal visible light emission.
- 🔥 The principles of quantum physics have been observable in everyday phenomena like fire for thousands of years, with flame colors indicating quantum effects.
Q & A
What was the initial problem Max Planck was asked to solve?
-Max Planck was asked to find a way to make light bulbs more efficient, ensuring they gave off the maximum light for the least electrical power.
What was the discrepancy Planck encountered when predicting the light emission of a hot filament?
-Planck's predictions based on electromagnetic theory were not aligning with experimental results, particularly concerning the amount of light emitted in different colors.
What revolutionary concept did Planck introduce to explain light emission?
-Planck introduced the concept that light waves carry energy in discrete packets, or 'quanta', with high frequency light consisting of large packets and low frequency light of small packets.
How did Albert Einstein relate Planck's quantum theory to a common problem?
-Einstein related the quantum theory to the problem of sharing a single cookie among multiple kids, illustrating how sharing energy packets among many entities leads to smaller portions.
What is the implication of having infinitely many small light waves in a room?
-The implication is that these light waves could theoretically consume all available energy, leading to a scenario where they could absorb all heat instantly, causing extreme cooling.
Why doesn't the universe behave as described in the 'infinitely many kids' analogy?
-The universe doesn't work that way because high frequency light waves can only carry energy in large packets, preventing the absorption of infinitesimally small amounts of energy.
What does the 'fussy' behavior of high frequency light waves mean in terms of energy distribution?
-High frequency light waves are selective in the amount of energy they carry, which means most of the energy is carried away by lower-frequency packets that are willing to share energy equally.
How is the average energy carried by light packets related to temperature?
-The average energy that the packets carry is what we refer to as 'temperature'. Therefore, a higher temperature corresponds to a higher average energy and a higher frequency of light emitted.
What temperature should a light bulb filament be heated to according to Planck's quantum theory?
-Planck's quantum theory suggests that a light bulb filament should be heated to about 3200 Kelvin to ensure that most of the energy is emitted as visible light.
How does the color of an object's glow change as it gets hotter?
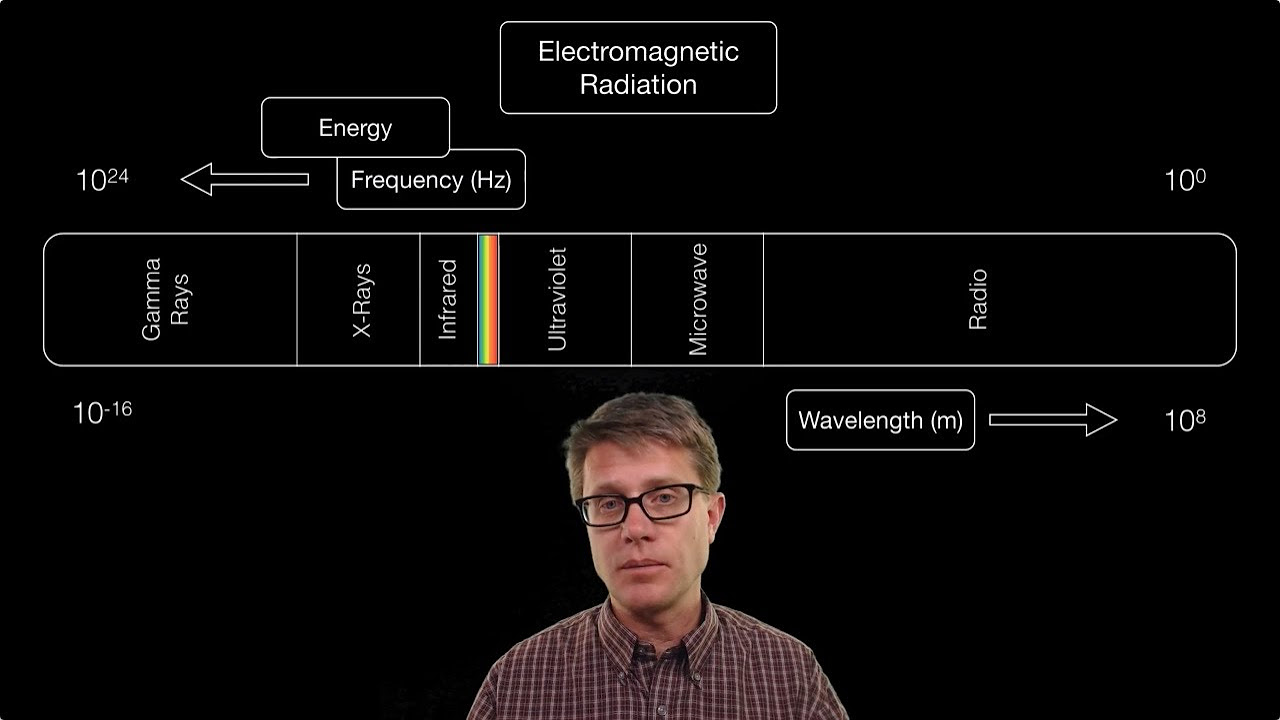
-As an object gets hotter, it glows in a sequence of colors from infrared to red, yellow, white, and then towards blue, violet, and ultraviolet as the temperature increases.
In what way have humans been experiencing quantum physics without realizing it?
-Humans have been experiencing quantum physics through the color changes in flames when making fires, which is a natural demonstration of quantum effects.
Outlines
💡 The Origin of Quantum Theory
Quantum theory originated from a practical problem: how to make light bulbs more efficient. Max Planck was tasked with this challenge and sought to predict the light output of a hot filament. He knew light was made up of electromagnetic waves of varying frequencies, corresponding to different colors. The goal was to maximize visible light while minimizing ultraviolet and infrared emissions. However, Planck's predictions based on electromagnetic theory were at odds with experimental results. In a desperate move, he disregarded the theory and derived a new rule from experimental data: light waves carry energy in discrete packets, with high-frequency light in larger packets and low-frequency light in smaller ones. This revolutionary idea, that light comes in quanta, was initially considered crazy but was later supported by Einstein's work on sharing and energy distribution. The analogy of sharing a single cookie among an increasing number of children illustrates the concept that infinitely small light waves could absorb all available energy, which would be disastrous. Fortunately, Planck's hypothesis that high-frequency light waves carry energy in large, specific packets prevents such an outcome. This leads to the conclusion that most energy is carried by lower-frequency light waves, which is related to the concept of temperature. As temperature increases, so does the average energy, resulting in higher frequencies of light emitted. This explains why objects glow in a sequence of colors as they heat up. Planck's quantum theory also suggests that light bulb filaments should be heated to about 3200 Kelvin for optimal visible light emission. The concept of quantum physics has been present in the form of fire for thousands of years, with the changing colors of flames indicating the underlying quantum nature.
Mindmap
Keywords
💡Quantum Theory
💡Max Planck
💡Electromagnetic Waves
💡Frequency
💡Energy Packets
💡Albert Einstein
💡Temperature
💡Black Body
💡
💡Efficiency
💡Visible Light
💡Infrared and Ultraviolet Light
💡Kelvin
Highlights
Quantum theory originated from a practical problem: increasing the efficiency of light bulbs.
Max Planck was tasked with predicting the light output of a hot filament.
Planck faced discrepancies between electromagnetic theory predictions and experimental results.
In an 'act of despair,' Planck discarded the existing theory and derived a new rule from experimental data.
The new rule proposed that light waves carry energy in discrete packets, known as 'quanta'.
Albert Einstein related the quantum theory to the concept of sharing, illustrating the limitations of infinite division.
Planck's theory implies that high-frequency light waves can only carry away energy in large packets.
The average energy carried by the packets is what we define as 'temperature'.
Higher temperature correlates with a higher average energy and a higher frequency of light emitted.
Planck's quantum theory explains why objects glow in a sequence of colors as they heat up.
According to Planck's theory, a light bulb filament should be heated to about 3200 Kelvin for optimal visible light emission.
Quantum physics has been an observable phenomenon in the color of fire flames for thousands of years.
Planck's work revolutionized the understanding of energy distribution in light waves.
The quantum theory has significant practical applications, such as in the design of light bulbs.
The concept of quanta was initially considered crazy but later became a fundamental principle of quantum physics.
Planck's approach to deriving a new rule of physics from experimental data was a significant shift from traditional theoretical physics.
The quantum theory's explanation of energy packets helps to prevent the paradox of infinite energy absorption by infinitesimal waves.
The quantum theory's insights into light and energy have profound implications for understanding the behavior of light at different frequencies.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: