Photons
TLDRIn this AP Physics essentials video, Mr. Andersen explains the concept of photons, the fundamental particles that constitute light. He discusses the photoelectric effect, a phenomenon where light ejects electrons from a metal surface, which was elucidated by Albert Einstein in 1905. Einstein's work revealed light's dual nature as both a particle and a wave, with photons carrying energy quantified by Planck's constant times the frequency of light. The video also touches on spectral lines as evidence for light's particle nature and the discrete energy levels in atoms. Viewers are encouraged to explore the photoelectric effect through a PHET simulation, demonstrating the linear relationship between photon energy and frequency, ultimately supporting the photon theory.
Takeaways
- 🌟 Photons are the fundamental particles that make up light, behaving as both waves and particles.
- 👨🏫 Albert Einstein explained the photoelectric effect in 1905, which demonstrated light's particle nature.
- 🔧 The photoelectric effect occurs when light, particularly UV light, ejects electrons from a metal surface.
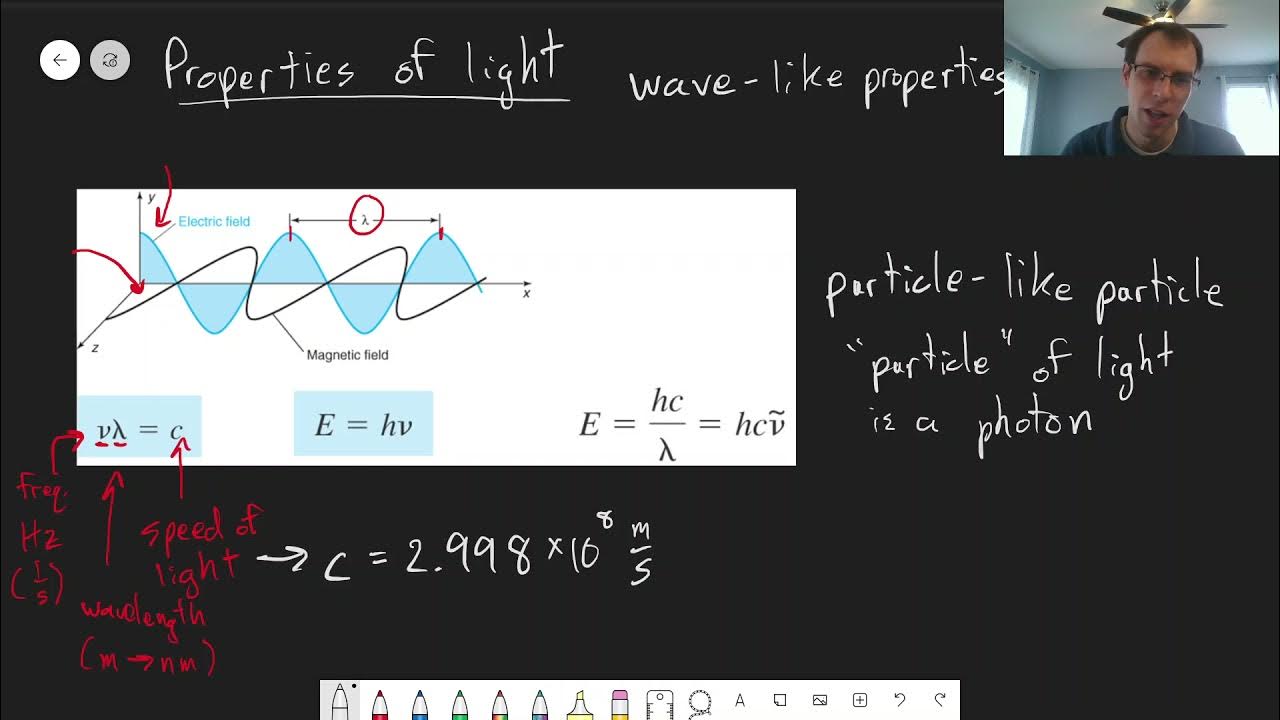
- 📐 The energy of a photon (E) is calculated using the formula E = hν, where h is Planck's constant and ν is the frequency of light.
- 📊 As the frequency of light increases, so does the energy of the photon.
- 🌈 Light exists across a spectrum, from high-energy gamma rays with short wavelengths to low-energy radio waves with long wavelengths.
- 🌈🌞 The spectral lines observed in hydrogen gas, both in emission and absorption, suggest that light comes in discrete packets or quanta.
- 🔄 Electrons in atoms absorb and emit photons when transitioning between energy levels.
- 📈 The photoelectric effect shows a linear relationship between the frequency of light and the energy released.
- 🛠️ The PHET simulation allows users to experiment with the photoelectric effect by measuring the current produced by ejected electrons and adjusting light intensity and wavelength.
- 🔑 Einstein's work on the photoelectric effect confirmed the quantized nature of light and helped establish the concept of photons.
Q & A
What are photons and why are they significant in the context of light?
-Photons are little packets that make up light, and they are significant because they exhibit both wave-like and particle-like properties, as explained by Albert Einstein. This dual nature is fundamental to understanding light's behavior.
What is the photoelectric effect, and how is it related to photons?
-The photoelectric effect is the phenomenon where electrons are emitted from a material when it is exposed to light. It is related to photons because the energy of the photons is what causes the electrons to be ejected from the material.
Who explained the photoelectric effect and when?
-Albert Einstein explained the photoelectric effect in 1905, which helped to establish the concept of light as being composed of particles, or photons.
What is Planck's constant (H), and how is it related to the energy of a photon?
-Planck's constant (H) is a fundamental physical constant that relates the energy of a photon to its frequency. The energy of a photon is equal to Planck's constant times the frequency of the light.
How does the frequency of light affect the energy of a photon?
-As the frequency of light increases, so does the energy of the photon. This is because the energy of a photon is directly proportional to the frequency of the light, as described by the formula E = H * frequency.
What does it mean for photons to be quantized?
-Quantized means that photons travel in discrete units rather than being spread out continuously along wavelengths. This implies that light energy comes in specific, indivisible packets.
What evidence supports the idea that light is quantized?
-Evidence supporting the quantization of light comes from spectral lines, both emission and absorption, which show that light exists in discrete units rather than a continuous spectrum.
What is the relationship between spectral lines and the quantization of light?
-Spectral lines, observed in emission and absorption spectra, are discrete lines that suggest light exists in discrete units or quanta. This is contrary to the idea of light as a continuous wave.
How does the energy level transition of an electron relate to the emission or absorption of a photon?
-When an electron moves to a higher energy level, it absorbs a photon, and when it falls back to a lower energy level, it emits a photon. This process shows that photons exist in discrete energy units.
What is the PHET simulation mentioned in the script, and how can it be used to understand the photoelectric effect?
-The PHET simulation is an interactive model that allows users to measure the electrons kicked off a metal surface when exposed to different light wavelengths and intensities. It helps to visualize and understand the photoelectric effect and how photon energy relates to electron ejection.
How does changing the wavelength of light affect the photoelectric effect?
-Changing the wavelength of light affects the frequency and, consequently, the energy of the photons. Decreasing the wavelength (increasing the frequency) can lead to the ejection of electrons if the photon energy is sufficient, demonstrating the direct relationship between frequency and energy.
What can be concluded from the linear relationship between frequency and energy in the context of the photoelectric effect?
-The linear relationship between frequency and energy, as demonstrated in the photoelectric effect, supports Einstein's theory that the energy of a photon is directly proportional to its frequency, confirming the quantized nature of light.
Outlines
🌟 Understanding Photons and the Photoelectric Effect
In this video script, Mr. Andersen introduces the concept of photons, which are the fundamental particles that constitute light. He explains the photoelectric effect, a phenomenon where light (specifically UV light) striking a metal surface can dislodge electrons, a concept first elucidated by Albert Einstein in 1905. Einstein's explanation not only clarified why light could eject electrons from metals but also introduced the idea that light behaves as both a wave and a particle. The script delves into the quantization of light, meaning it exists in discrete packets or photons, and discusses Planck's constant (H) and its relationship with the energy of a photon, which is given by the formula H = Planck’s constant * frequency. The video also touches upon the electromagnetic spectrum, highlighting the range from high-energy gamma rays to low-energy radio waves, and the historical model of light as a continuous transverse wave before the discovery of spectral lines.
Mindmap
Keywords
💡Photons
💡Photoelectric Effect
💡Albert Einstein
💡Planck’s Constant (H)
💡Frequency
💡Spectral Lines
💡Electromagnetic Radiation
💡Gamma Rays
💡Radio Waves
💡Transverse Wave
💡Electron
Highlights
Photons are described as little packets that make up light.
The photoelectric effect is explained, where light can kick off electrons.
Albert Einstein's explanation of the photoelectric effect in 1905.
Light behaves as both a wave and a particle.
Einstein's equation E=hν, where E is the energy of a photon, h is Planck’s constant, and ν is the frequency of light.
Quantization of photons, meaning they travel in discrete units.
Spectral lines as evidence for the discrete nature of light.
The model of light as an electromagnetic transverse wave prior to Einstein.
Discrete spectral emission lines from hydrogen suggest light exists in discrete units.
Electrons absorb and emit photons when moving between energy levels.
Different atoms require varying photon amounts for energy level transitions.
The PHET simulation as a tool to measure electron ejection from metal due to light.
Observation that no electrons are ejected with infrared light, but a sudden increase occurs with higher frequencies.
Linear relationship between frequency and energy in the photoelectric effect.
Demonstration of the direct relationship between frequency and energy using copper and zinc plates.
Einstein's conclusion that the energy of an individual photon is directly calculable using the frequency times Planck’s constant.
The photoelectric effect as evidence supporting the photon theory.
The educational value of the video in understanding the photon theory and the photoelectric effect.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: