Double Displacement Reaction Experiment
TLDRThis educational video script introduces viewers to the concept of a double displacement reaction through a hands-on experiment. The process involves using barium chloride and sodium sulfate solutions, mixed in test tubes with water. After shaking the mixture, the addition of dilute sulfuric acid reveals the formation of an insoluble white precipitate, barium sulfate, and the common table salt, sodium chloride. The experiment visually demonstrates the exchange of ions between the two salts, resulting in the formation of two new compounds. This engaging summary invites viewers to explore the principles of chemical reactions and the fascinating outcomes of double displacement.
Takeaways
- 🧪 The experiment demonstrates a double displacement reaction.
- 🔬 Three test tubes and a stand are required for the experiment setup.
- 💧 Barium chloride and sodium sulfate are the two salts used in the experiment.
- 🚰 Water is added to both test tubes to dissolve the salts.
- 🌀 The solutions are shaken to ensure proper mixing.
- 🔁 The sodium sulfate solution is added to the barium chloride solution.
- ⏳ The reaction requires time to form the end products.
- 🚨 Excess water is removed before adding dilute sulfuric acid.
- 📏 A third test tube is used to store the removed excess water.
- 🌟 The addition of dilute sulfuric acid helps to identify the precipitate formed.
- ⚗️ An insoluble white precipitate of barium sulfate is formed.
- 🔄 The reaction involves the exchange of ions between two salts.
- 🧂 The other product of the reaction is sodium chloride, common table salt.
Q & A
What is a double displacement reaction?
-A double displacement reaction is a type of chemical reaction where the ions of two compounds exchange places, resulting in the formation of two new compounds.
What materials are needed to perform the experiment described in the transcript?
-To perform the experiment, you will need three test tubes, a test tube stand, barium chloride salt, sodium sulfate solution, water, and dilute sulfuric acid.
How much water is added to each test tube in the experiment?
-Approximately 5 ml of water is added to each test tube.
What happens when sodium sulfate solution is added to the barium chloride solution?
-The solution turns completely white, indicating that a reaction has occurred between the two compounds.
What is the purpose of shaking the test tubes after adding the solutions?
-Shaking the test tubes helps to mix the solutions properly, ensuring a thorough chemical reaction takes place.
Why is dilute sulfuric acid added to the mixture after some time?
-Dilute sulfuric acid is added to observe the formation of a precipitate, which is a key indicator of the products formed in a double displacement reaction.
What is the role of the third test tube in the experiment?
-The third test tube is used to store the excess water removed from the reaction mixture, allowing for a clearer observation of the precipitate formed.
What is the white precipitate formed in the reaction?
-The white precipitate formed is barium sulfate, which is insoluble in water.
What are the two salts formed as a result of the double displacement reaction in the experiment?
-The two salts formed are barium sulfate precipitate and sodium chloride, which is a common table salt.
How does the formation of barium sulfate and sodium chloride demonstrate a double displacement reaction?
-In a double displacement reaction, the ions in the reactant compounds exchange partners to form two new compounds. In this case, the sulfate ions from sodium sulfate replace the chloride ions in barium chloride, and vice versa, forming barium sulfate and sodium chloride.
Why is the term 'displacement' used in the context of a double displacement reaction?
-The term 'displacement' refers to the process where one ion in a compound is replaced by another ion. In a double displacement reaction, this occurs twice, hence the name, as two different ions are exchanged between two compounds.
What is the significance of observing a precipitate in a double displacement reaction?
-The formation of a precipitate is significant as it indicates that a new compound has been formed that is insoluble in the reaction medium. In the context of the experiment, the formation of barium sulfate precipitate confirms the occurrence of the double displacement reaction.
Outlines
🧪 Double Displacement Reaction Experiment Overview
This paragraph introduces the concept of a double displacement reaction, explaining that the experiment will demonstrate this type of chemical reaction. The setup includes three test tubes, a test tube stand, barium chloride salt, and sodium sulfate solution. The process involves adding these substances to separate test tubes, followed by the addition of water. The paragraph concludes with the intention to mix the solutions and observe the reaction's outcome after adding dilute sulfuric acid.
Mindmap
Keywords
💡Double Displacement Reaction
💡Test Tubes
💡Barium Chloride
💡Sodium Sulfate
💡Water
💡Shaking
💡Precipitate
💡Dilute Sulfuric Acid
💡Ions
💡Sodium Chloride
💡Chemical Reaction
Highlights
Introduction to the concept of a double displacement reaction through an experiment.
Necessity of three test tubes and a test tube stand for the experiment setup.
Use of barium chloride salt and sodium sulfate solution in the experiment.
Addition of 5 ml of water to each test tube containing the salts.
Shaking the test tubes to mix the solutions thoroughly.
Combining sodium sulfate solution with barium chloride solution to initiate the reaction.
Observation of the solution turning white, indicating the start of the reaction.
The process of waiting and then removing excess water to observe the end product.
Introduction of dilute sulfuric acid to reveal the formation of a precipitate.
Use of a third test tube to store the excess water removed from the reaction mixture.
Formation of an insoluble white precipitate identified as barium sulfate.
Explanation of the chemical process where chloride ions are replaced by sulfate ions in barium chloride.
Formation of two new salts in the reaction: barium sulfate precipitate and sodium chloride.
Description of the double displacement reaction where two ions are exchanged between two compounds.
The significance of the experiment in understanding ionic exchange and chemical reactions.
Visual demonstration of the reaction process using a dropper for mixing.
Educational value of the experiment in illustrating the principles of chemistry.
Thanking the viewers for watching and engaging with the experiment.
Transcripts
Browse More Related Video

Writing Net Ionic Equations - AP Chemistry Unit 4, Topic 2b
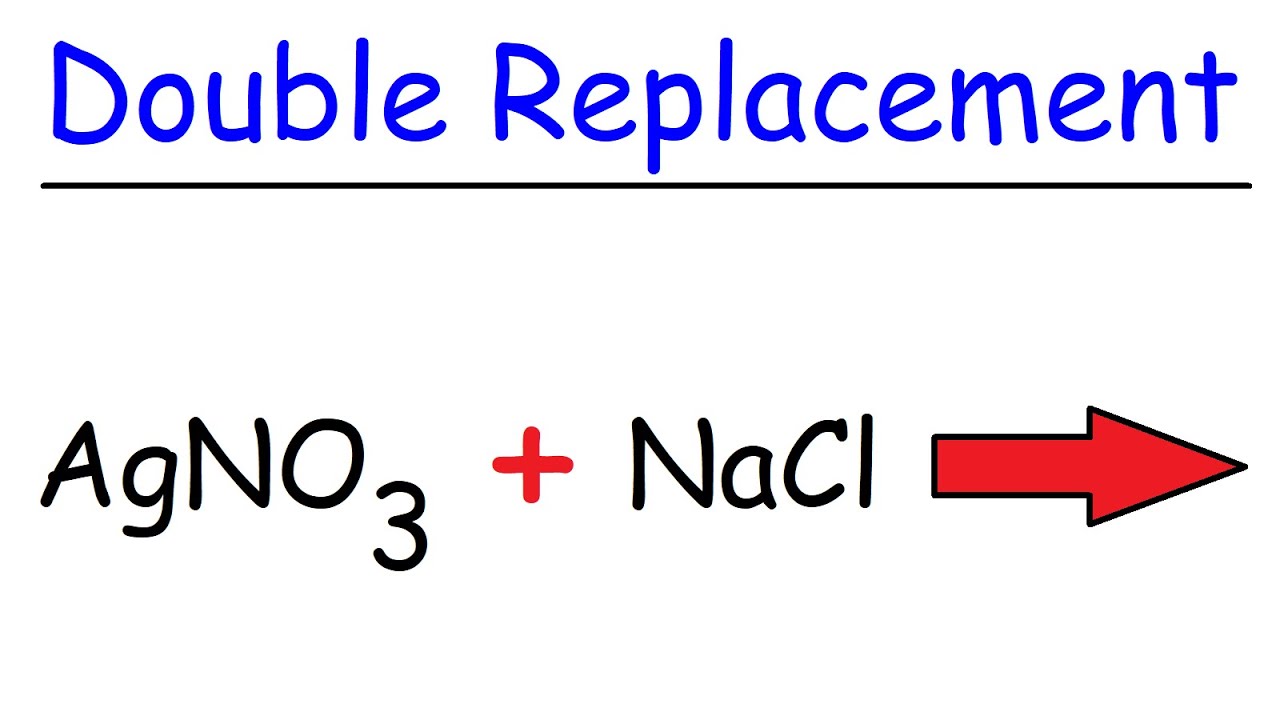
Introduction to Double Replacement Reactions

Chemical Reactions (1 of 11) Double Replacement Reactions, An Explanation

5 Types of Chemical Reactions (Chemistry) + Activity Series, Solubility Rules
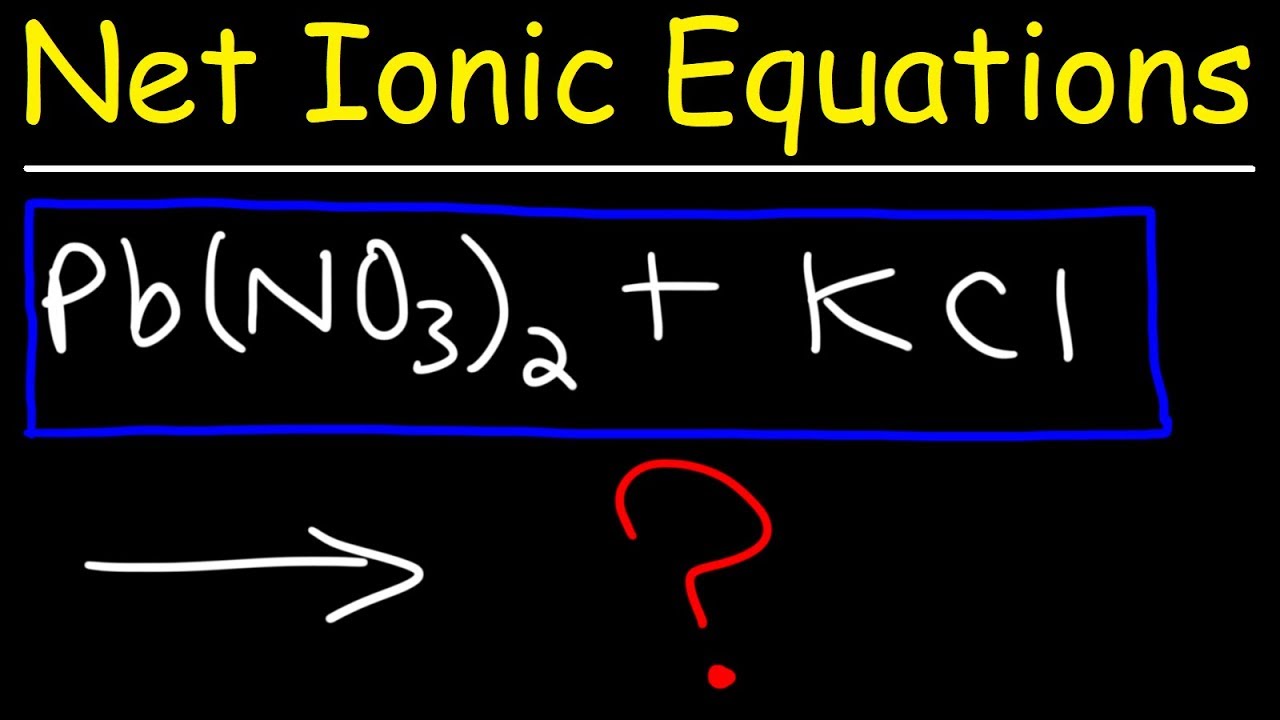
How To Write Net Ionic Equations In Chemistry - A Simple Method!
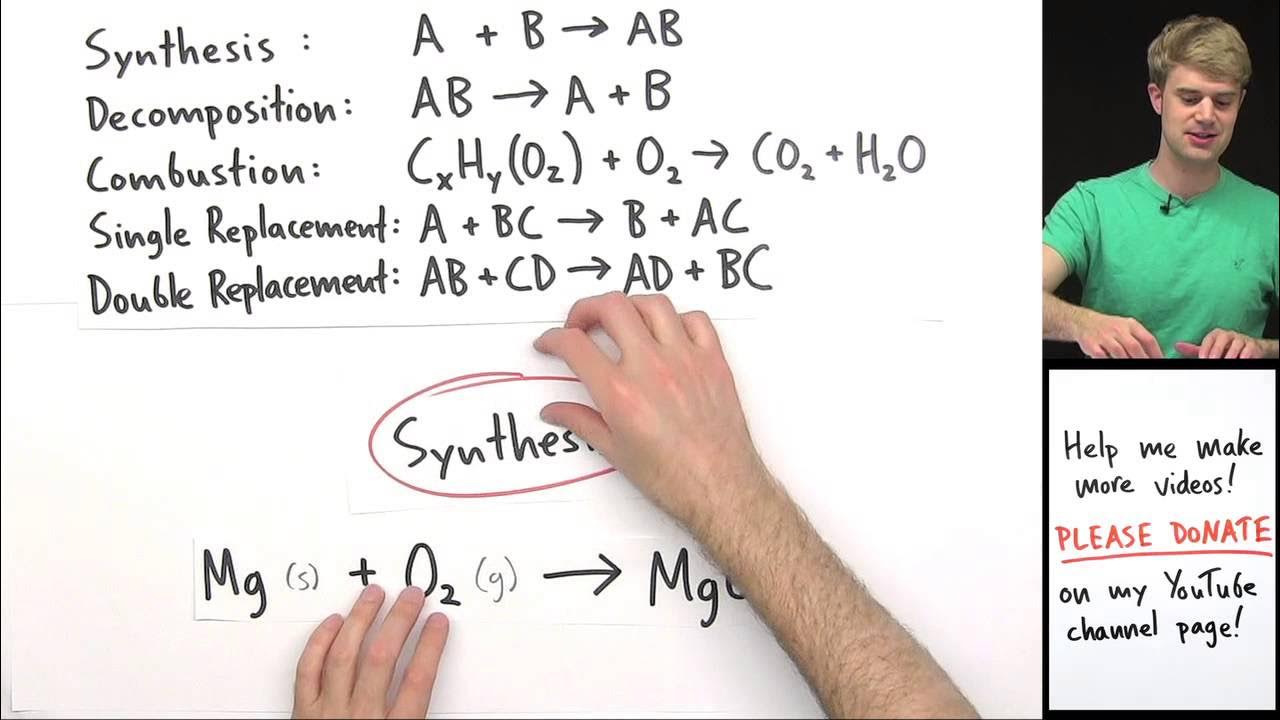
Classifying Types of Chemical Reactions Practice Problems
5.0 / 5 (0 votes)
Thanks for rating: