5 Types of Chemical Reactions (Chemistry) + Activity Series, Solubility Rules
TLDRThe video script delves into the fascinating world of chemical reactions, explaining the five basic types: synthesis, decomposition, single-displacement, double-displacement, and combustion. It describes synthesis as the combination of simpler reactants into a complex product, exemplified by rusting iron. Decomposition breaks compounds into smaller parts, as seen in water electrolysis. Single-displacement involves ions swapping places, like copper and silver nitrate. Double-displacement reactions involve two sets of ions exchanging partners, often resulting in precipitation, as in the reaction between sodium hydroxide and calcium chloride. Lastly, combustion involves a substance reacting with oxygen, producing carbon dioxide and water, and is illustrated with propane combustion. The script also provides practical tips for preventing rust and the importance of the metal activity series in predicting reactions. It concludes with an offer of a practice test for those studying chemical reactions, available on socratica.com.
Takeaways
- 🔬 A chemical reaction involves a change in the chemical composition of a substance, which can be identified by signs like color change or gas release.
- 🔥 Chemical reactions can range from dramatic events like fires and explosions to slow processes like the formation of fossil fuels over millions of years.
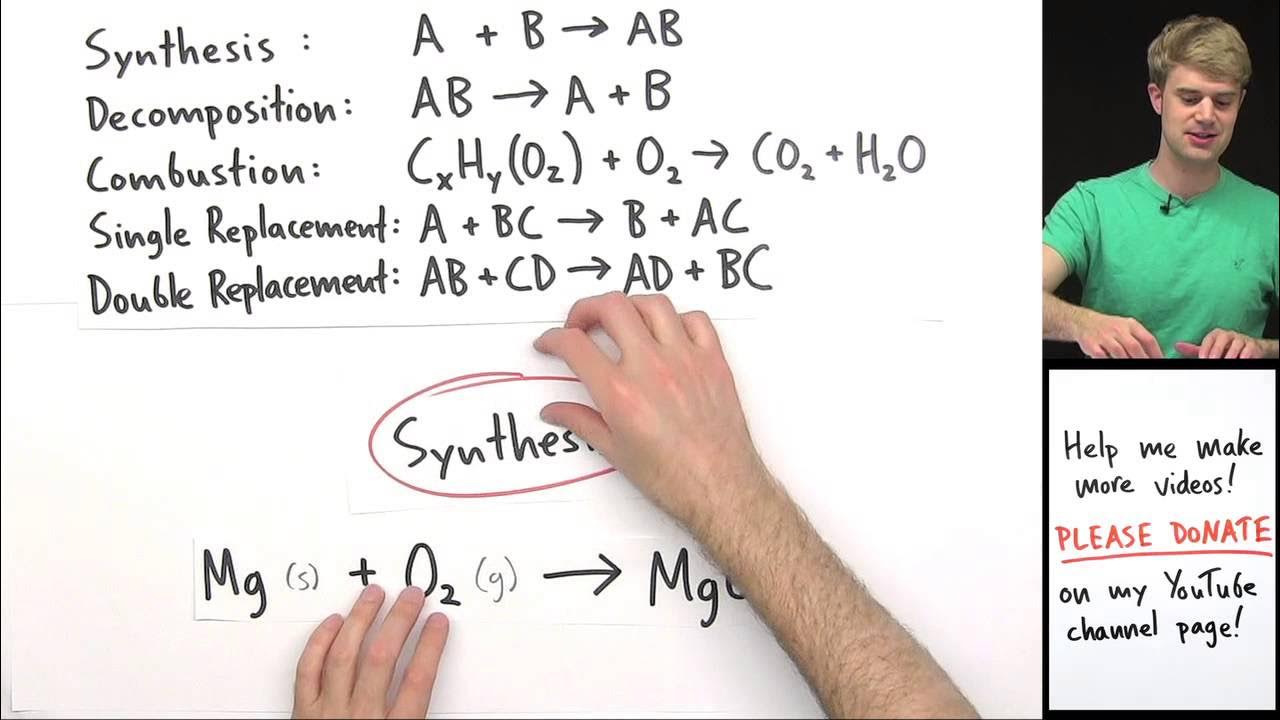
- 📚 Chemists categorize reactions into five basic types: synthesis, decomposition, single-displacement, double-displacement, and combustion.
- 🤝 Synthesis reactions, also known as combination reactions, involve combining two or more simpler reactants into a larger, more complex product (A + B → AB).
- ⚡ Decomposition reactions break a compound into smaller pieces, requiring an input of energy, and are sometimes called 'analysis reactions' (AB → A + B).
- 🔄 Single-displacement reactions involve one ion swapping places with another, often occurring when metals are placed in an aqueous solution (A + BC → AC + B).
- 🤲 Double-displacement reactions involve two sets of components swapping places to form two new compounds, often resulting in the formation of a precipitate (AB + CD → AD + CB).
- 🧪 Precipitation reactions are a specific kind of double-displacement reaction where two ions form an insoluble compound that precipitates out of solution.
- 🌦️ Acid-base reactions, such as neutralization, are a type of double-displacement reaction that does not form a precipitate but produces water and a salt.
- 🔥 Combustion reactions involve a substance reacting with oxygen, typically releasing carbon dioxide and water, and are commonly associated with burning hydrocarbons (hydrocarbon + O2 → CO2 + H2O).
- 🧑🔬 The metal activity series and halogen activity series are used to predict whether one metal or halogen will displace another in a reaction.
- 📈 Balancing chemical equations can be done using the inspection method or the algebraic method, and understanding these methods is crucial for predicting reaction products.
Q & A
What is a chemical reaction?
-A chemical reaction is a process that causes a change in the chemical composition of a substance, which can be indicated by signs such as color change or gas release.
What are the five basic types of chemical reactions?
-The five basic types of chemical reactions are synthesis, decomposition, single-displacement, double-displacement, and combustion.
What is a synthesis reaction and what is its general form?
-A synthesis reaction, also known as a combination reaction, involves combining two or more simpler chemical reactants into one larger, more complex product. Its general form is A + B → AB.
How does rusting relate to a synthesis reaction?
-Rusting is an example of a synthesis reaction where iron (metal) reacts with oxygen to form a new compound, iron(III) oxide, which is a combination of the two elements.
What is the general form of a decomposition reaction and an example of such a reaction?
-The general form of a decomposition reaction is AB → A + B. An example is the electrolysis of water to form hydrogen gas and oxygen gas.
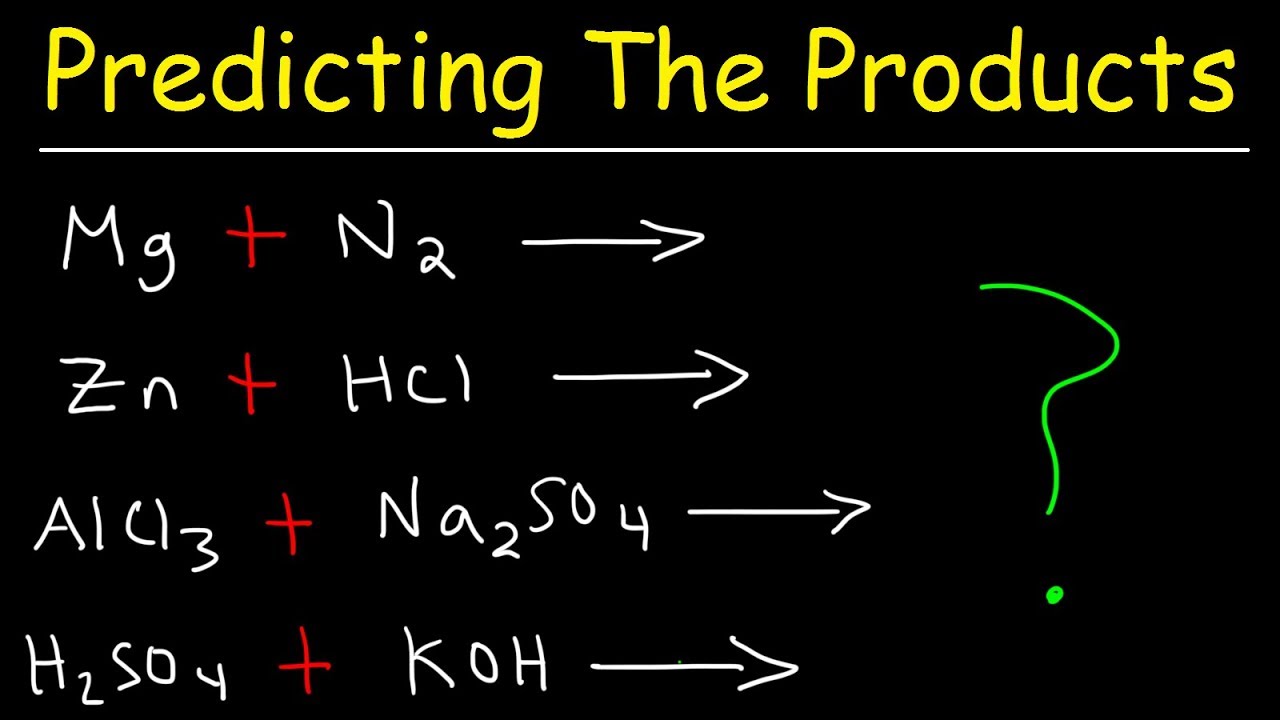
How does the metal activity series help predict the outcome of a single-displacement reaction?
-The metal activity series is a table of data that allows chemists to predict whether one metal will displace another in a single-displacement reaction. Metals higher in the activity series can replace those lower down.
What is the general form of a single-displacement reaction?
-The general form of a single-displacement reaction is A + BC → AC + B, where elements A and B swap places.
What is the difference between a double-displacement reaction and a precipitation reaction?
-A double-displacement reaction involves two sets of components swapping places, with the general form AB + CD → AD + CB. A precipitation reaction is a specific kind of double-displacement reaction that results in the formation of a precipitate, an insoluble compound in water.
How do you determine if a precipitate will form in a double-displacement reaction?
-To determine if a precipitate will form, you use a table of solubility rules, which provides information on the solubility of different ionic compounds in water.
What is the general form of a combustion reaction and an example of such a reaction?
-The general form of a combustion reaction is hydrocarbon + O2 → CO2 + H2O. An example is the combustion of propane, with the balanced chemical equation being C3H8 + 5O2 → 3CO2 + 4H2O.
Why are combustion reactions of interest to humans?
-Combustion reactions are of interest because they involve substances reacting with oxygen, often releasing energy in the form of heat and light, which humans have used for cooking, transportation, and as a source of fascination.
What is the role of the metal activity series and solubility rules in predicting chemical reactions?
-The metal activity series and solubility rules are essential tools for predicting the outcomes of chemical reactions, particularly in displacement and double-displacement reactions. They provide a systematic way to determine whether a reaction will occur and what products will be formed.
Outlines
🔍 Understanding Chemical Reactions
This paragraph introduces the concept of chemical reactions, which are processes that alter the chemical composition of substances. It explains that there are signs to look for, such as color changes or gas release, and that reactions can range from dramatic (like fires) to slow (like fossil fuel formation). Chemists categorize reactions into five main types: synthesis, decomposition, single-displacement, double-displacement, and combustion. The paragraph also mentions that some reactions can be further classified, like precipitation and neutralization reactions. The goal is to classify reactions to predict their outcomes and duration, using real-life examples for each type.
🧪 Types of Chemical Reactions: Synthesis and Decomposition
The first type of reaction discussed is synthesis, also known as a combination reaction, where two or more simple reactants combine to form a more complex product. An example given is rusting, where iron reacts with oxygen to form iron(III)oxide, which is a synthesis of iron and oxygen. The paragraph also covers decomposition reactions, which are the reverse of synthesis, where a compound breaks down into simpler substances. Examples include the electrolysis of water and the decomposition of hydrogen peroxide into water and oxygen, which can occur without external energy input.
🔋 Single-Displacement, Double-Displacement, and Other Reactions
The third paragraph covers single-displacement reactions, where one ion swaps places with another, often occurring when metals are placed in an aqueous solution. An example is copper displacing silver in a silver nitrate solution. The metal activity series table is used to predict such displacements. The paragraph also explains double-displacement reactions, where two sets of components exchange parts, often resulting in the formation of a precipitate. Precipitation reactions are a specific type of double-displacement reaction where an insoluble compound forms. Solubility rules are used to predict precipitates. The paragraph concludes with a discussion on reactions that produce a gas instead of a precipitate, such as the reaction between sodium bicarbonate and HCl, and acid-base neutralization reactions.
🔥 Combustion Reactions and Practical Advice for Students
The final paragraph focuses on combustion reactions, where a substance reacts with oxygen, typically producing carbon dioxide and water. It provides examples like the combustion of propane and hydrogen gas. The paragraph emphasizes that combustion doesn't always involve hydrocarbons, as demonstrated by the hydrogen gas example. It concludes with advice for students preparing for tests on chemical reactions, recommending the use of practice tests to identify knowledge gaps. A practice test with an answer key is available for purchase on the website socratica.com, with the proceeds supporting the creation of free educational content.
Mindmap
Keywords
💡Chemical reaction
💡Synthesis reaction
💡Decomposition reaction
💡Single-displacement reaction
💡Double-displacement reaction
💡Combustion
💡Metal activity series
💡Halogen activity series
💡Solubility rules
💡Neutralization reaction
💡Precipitation reaction
Highlights
Chemical reactions involve a change in the chemical composition of a substance and can be identified by signs like color change or gas release.
Chemical reactions can range from dramatic events like fires to slow processes like the formation of fossil fuels.
Chemists classify reactions into five basic types: Synthesis, Decomposition, Single-displacement, Double displacement, and Combustion.
Synthesis reactions combine two or more reactants into a larger, more complex product.
Rusting, a common process, is actually a synthesis reaction where iron reacts with oxygen to form iron(III)oxide.
Decomposition reactions break a compound into smaller pieces and often require an input of energy.
Electrolysis of water is an example of a decomposition reaction that requires electrical energy to break water into hydrogen and oxygen.
Single-displacement reactions involve the swapping of ions, where one ion trades places with another.
A metal activity series table is used to predict whether one metal will displace another in a single-displacement reaction.
Double-displacement reactions involve two sets of components swapping places to form two new compounds.
Precipitation reactions are a specific kind of double-displacement reaction where an insoluble compound forms.
Neutralization reactions are a type of double-displacement reaction where an acid and a base react to form water and a salt.
Combustion reactions involve a substance reacting with oxygen, typically releasing carbon dioxide and water.
The Hindenburg accident is an example of a combustion reaction involving hydrogen gas, which is highly flammable.
Socratica provides a practice test with an answer key for those studying chemical reactions, available on their website.
The metal activity series and solubility rules are essential tools for predicting outcomes in single-displacement and double-displacement reactions.
Halogens, like metals, have an activity series that determines their ability to participate in single-displacement reactions.
Not all double-displacement reactions result in a precipitate; some can produce a gas, as seen in the reaction between sodium bicarbonate and HCl.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: