Molality and Colligative Properties
TLDRIn this informative video, Professor Dave delves into the concept of colligative properties, which are physical characteristics of a solution that depend solely on the concentration of solute rather than its chemical identity. He explains how the presence of solute particles in a solvent affects phase changes, leading to three key phenomena: vapor pressure lowering, boiling point elevation, and freezing point depression. To quantify these effects, the video introduces molality, a measure of solute concentration in relation to the mass of solvent. Professor Dave illustrates how solute particles interfere with the solvent's activity at phase interfaces, causing a decrease in vapor pressure and an elevation in boiling point, while also explaining the principle behind freezing point depression. He emphasizes the practical application of these properties, such as the use of salt to prevent ice formation on streets. The video concludes with an invitation to subscribe for more educational content and an offer to answer questions via email.
Takeaways
- 🌡️ Colligative properties are physical characteristics of a solution that depend on the concentration of solute, not its chemical identity.
- 🔍 Adding solute to a solvent affects vapor pressure, boiling point, and freezing point differently from a pure solvent.
- 📊 Molality is a measure of solute concentration used in colligative properties, expressed as moles of solute per kilogram of solvent.
- 💧 Solute particles at the liquid surface reduce the vapor pressure by occupying surface area and hindering solvent evaporation.
- 🔥 Boiling point elevation occurs because solute particles block solvent molecules from vaporizing, requiring more heat energy.
- 🧊 Freezing point depression happens when solute particles interfere with the solvent's ability to form a lattice, necessitating a lower temperature to freeze.
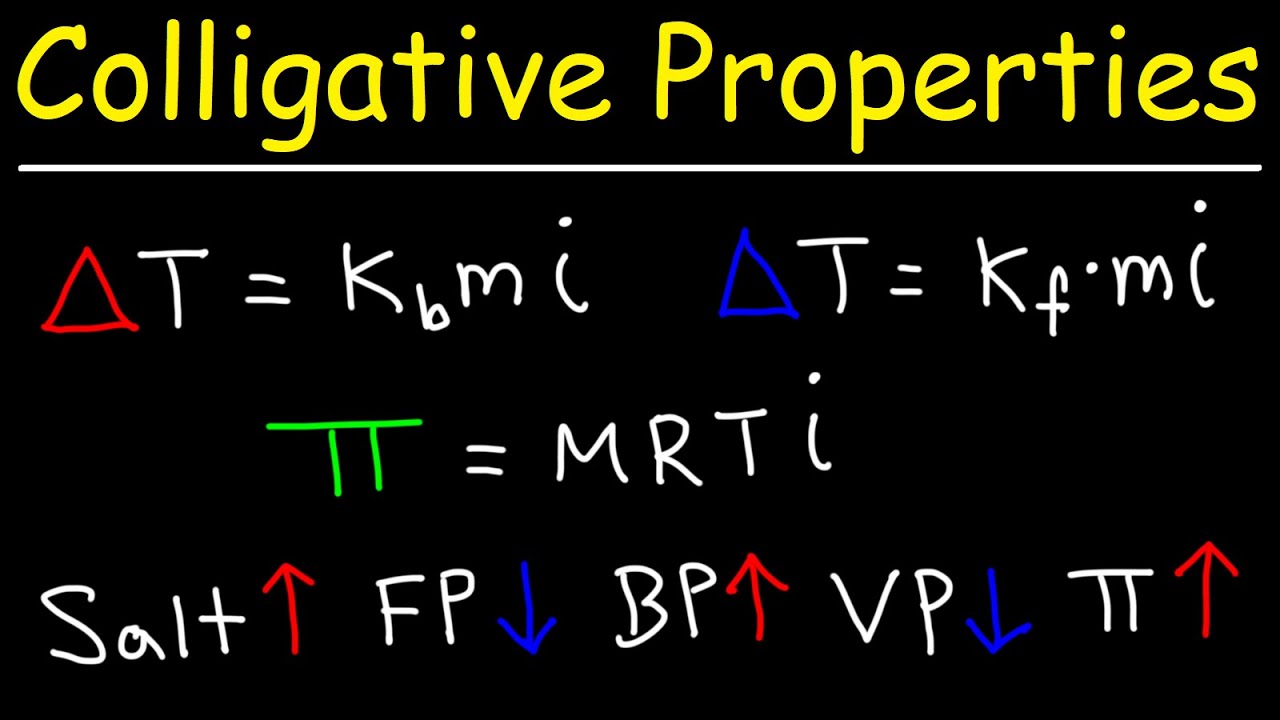
- 📏 The change in boiling point is calculated by multiplying the solution's molality by a solvent-specific constant (Kb).
- ❄️ Similarly, the change in freezing point is found by multiplying the solution's molality by another solvent-specific constant (Kf).
- ➕ The boiling point of a solution is always higher than that of the pure solvent, so you add the change to the original boiling point.
- ➖ The freezing point of a solution is always lower than that of the pure solvent, so you subtract the change from the original freezing point.
- ⛄️ Salt is added to icy streets to lower the freezing point of water, keeping it liquid at lower temperatures and reducing ice formation.
- 📚 Kb and Kf constants for different solvents can be found in textbooks or online resources.
Q & A
What are colligative properties?
-Colligative properties are characteristics of a solution that depend only on the concentration of solute and not on the chemical identity of the solute particles. They include vapor pressure lowering, boiling point elevation, and freezing point depression.
How does the presence of solute particles affect the vapor pressure of a liquid?
-Solute particles at the surface of the liquid occupy some of the surface area, which hinders solvent molecules from evaporating. This causes the vapor pressure of the liquid to decrease.
What is molality and how is it different from molarity?
-Molality is a measure of solute concentration expressed as moles of solute per kilogram of solvent. It differs from molarity, which is moles of solute per liter of solution.
How does the molality of a solution influence its boiling point?
-The boiling point elevation is directly related to the molality of the solution. The more solute there is, the more heat energy is needed to overcome the solute particles' interference, resulting in a higher boiling point.
What is the effect of solute particles on the freezing point of a solvent?
-Solute particles interfere with the solvent particles' ability to form a lattice structure, which is necessary for freezing. This requires the system to reach a lower temperature to freeze, thus causing a depression in the freezing point.
What are the Kb and Kf constants, and where can they be found?
-The Kb and Kf constants are specific to the solvent and are used in the equations for calculating the change in boiling and freezing points, respectively. They can be found in tables in textbooks or online.
Why do we add salt to icy streets?
-Salt is added to icy streets because it lowers the freezing point of water, causing it to remain liquid at lower temperatures. This reduces the amount of ice that forms and helps to melt existing ice.
How do colligative properties affect the phase changes of a solvent?
-Colligative properties affect phase changes by altering the physical processes that occur at the interface between two phases. The presence of solute particles interferes with the solvent's ability to change phases, leading to changes in vapor pressure, boiling point, and freezing point.
What is the relationship between the mole fraction of the solvent and the new vapor pressure of a solution?
-The new vapor pressure of a solution is equal to the vapor pressure of the pure solvent times the mole fraction of the solvent, which is the percentage of particles in the solution that are solvent molecules.
How does the molality of a solution relate to the change in boiling and freezing points?
-The change in boiling point is given by the molality of the solution times the Kb constant, while the change in freezing point is given by the molality times the Kf constant. These constants are specific to the solvent.
Why is it important to use molality instead of molarity when discussing colligative properties?
-Molality is preferred over molarity for colligative properties because it accounts for the mass of the solvent, which is more relevant when discussing the effect of solute concentration on phase changes.
Can you provide an example of how to calculate the molality of a solution?
-Sure. If you have a solution with 10 grams of iodine in 30 grams of dichloromethane, you would first convert the grams of iodine to moles (using the molar mass of iodine) and then divide by the mass of the solvent in kilograms to find the molality.
Outlines
🔬 Colligative Properties and Solute Concentration
Professor Dave introduces colligative properties, which are the behaviors of a solution that differ from a pure solvent due to the presence of solute particles. These properties include vapor pressure lowering, boiling point elevation, and freezing point depression, and they depend solely on the solute's concentration, not its chemical identity. To discuss these properties, the concept of molality is introduced, which measures moles of solute per kilogram of solvent. An example is given with iodine in dichloromethane to illustrate how to calculate molality. The key theme is that solute particles interfere with the solvent's activity at phase interfaces.
Mindmap
Keywords
💡Colligative properties
💡Solute
💡Solvent
💡Molality
💡Vapor pressure lowering
💡Boiling point elevation
💡Freezing point depression
💡Mole fraction
💡Kb and Kf constants
💡Salt
💡Phase changes
Highlights
Colligative properties describe how a solution behaves differently from a pure solvent due to the presence of solute particles.
Colligative properties depend only on the concentration of solute, not on the chemical identity of the solute particles.
Adding solute to a solvent affects vapor pressure, boiling point, and freezing point.
Molality is used to express solute concentration in colligative properties, defined as moles solute per kilogram of solvent.
Molality is denoted with a lowercase 'm' in italics, unlike molarity which uses an uppercase 'M'.
Solute particles interfere with the activity of solvent particles at the interface between phases.
The presence of solute in a liquid decreases its vapor pressure by occupying surface area and hindering solvent evaporation.
The new vapor pressure is calculated as the vapor pressure of the pure solvent times the mole fraction of the solvent.
Boiling point elevation occurs because solute particles block solvent molecules from entering the gas phase, requiring more heat energy.
The change in boiling point is calculated by multiplying the solution's molality by a solvent-specific constant.
Freezing point depression happens when solute particles interfere with the solvent's ability to form a lattice, requiring a lower temperature to freeze.
The change in freezing point is given by the solution's molality times another constant, which can be found in textbooks or online.
Kb and Kf constants are used in the equations for boiling point elevation and freezing point depression, respectively.
The boiling point is always increased by the addition of solute, while the freezing point is decreased.
Salt is added to icy streets to lower the freezing point of water, keeping it liquid at lower temperatures and reducing ice formation.
The practical application of colligative properties is demonstrated through the use of salt on icy streets.
Understanding colligative properties is essential for various scientific and industrial processes involving solutions.
Transcripts
Browse More Related Video
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
13.3 Colligative Properties | General Chemistry

11.3 Colligative Properties | High School Chemistry

Boiling point elevation and freezing point depression | Chemistry | Khan Academy

Lec-05 I Colligative properties I Applied Chemistry I Chemical Engineering

Molarity , Molality or Mole Fraction || 3D animated explanation || solutions || class12thchemistry
5.0 / 5 (0 votes)
Thanks for rating: