13.3 Colligative Properties | General Chemistry
TLDRThis chemistry lesson delves into colligative properties, focusing on the van 't Hoff factor and its impact on properties like freezing point depression, boiling point elevation, vapor pressure depression, and osmotic pressure. The instructor, Chad, explains how solute concentration affects these properties and provides formulas for calculations. He clarifies misconceptions about boiling points and introduces Raoult's Law. The lesson aims to give students a solid understanding of how to calculate and predict changes in colligative properties.
Takeaways
- 🌡 Colligative properties are physical properties of a solution that depend on the concentration of solute particles, not the chemical identity of the solute.
- 🧪 The van 't Hoff factor (i) is a key concept in understanding colligative properties, representing the number of particles a solute dissociates into in solution.
- ❄️ Freezing point depression occurs when the addition of a solute to a solvent lowers the temperature at which the solution freezes.
- 🔥 Boiling point elevation happens when the presence of a solute causes the solution to boil at a higher temperature than the pure solvent.
- 💧 Raoult's law describes the relationship between the mole fraction of a solvent in a solution and its vapor pressure, leading to vapor pressure depression.
- 📉 Osmotic pressure (π) is the pressure that needs to be applied to prevent the flow of water across a semi-permeable membrane due to differences in solute concentration.
- 🔍 The van 't Hoff factor is crucial for calculating the molality of particles in solutions involving electrolytes, which affects colligative properties.
- 📚 Strong electrolytes, like NaCl, dissociate into ions and have a van 't Hoff factor equal to the number of ions produced, while non-electrolytes have a factor of one.
- 📉 The change in freezing and boiling points can be calculated using the constants specific to the solvent and the molality of solute particles.
- 🌡 The actual physical reasons behind the changes in freezing and boiling points involve the disruption of the solvent's crystal lattice and the energy balance between liquid and gas phases.
- 🚰 Understanding osmotic pressure is essential for processes like reverse osmosis, where pressure is applied to drive water against its natural flow to purify it.
Q & A
What are colligative properties?
-Colligative properties are properties of a solution that depend on the concentration of solute particles, rather than the nature of the solute itself. They include freezing point depression, boiling point elevation, vapor pressure depression, and osmotic pressure.
What is the van 't Hoff factor and why is it important?
-The van 't Hoff factor accounts for the number of particles a solute dissociates into when dissolved in a solution. It is important because it affects the concentration of particles in the solution, which in turn influences the extent to which colligative properties change.
How does the identity of the solute affect colligative properties?
-The identity of the solute does not significantly affect colligative properties. It is the concentration and number of solute particles that matter, not the chemical identity of the solute.
What is the relationship between the van 't Hoff factor and the concentration of particles in a solution?
-The van 't Hoff factor directly impacts the concentration of particles in a solution. For non-electrolytes, the factor is 1, meaning they do not dissociate. For electrolytes, the factor is the number of ions they dissociate into, which increases the particle concentration.
How does the freezing point of a solution change with the addition of a solute?
-The freezing point of a solution decreases with the addition of a solute. This is due to the increased number of particles disrupting the intermolecular forces, making it more difficult for the solvent to form a crystalline structure.
What is Raoult's Law and how does it relate to vapor pressure depression?
-Raoult's Law states that the partial vapor pressure of a component in a solution is equal to the mole fraction of that component multiplied by its vapor pressure when pure. It relates to vapor pressure depression by showing that the presence of a non-volatile solute reduces the vapor pressure of the solvent above the solution.
How does the boiling point of a solution change with the addition of a solute?
-The boiling point of a solution increases with the addition of a solute. This is because the solute lowers the energy of the solution, requiring additional heat to reach the point where the chemical potentials of the liquid and gas phases are equal.
What is osmotic pressure and how is it calculated?
-Osmotic pressure is the pressure that needs to be applied to prevent the flow of water across a semi-permeable membrane due to differences in solute concentration. It is calculated using the formula π = iMRT, where i is the van 't Hoff factor, M is the molarity, R is the ideal gas constant, and T is the temperature in Kelvin.
How does the concentration of solute affect the colligative properties of a solution?
-The concentration of solute directly affects the colligative properties of a solution. As the concentration of solute increases, the changes in freezing point, boiling point, and osmotic pressure also increase due to the greater number of particles affecting the solvent's behavior.
What is the significance of the van 't Hoff factor in calculating the boiling point elevation of a solution?
-The van 't Hoff factor is used to determine the effective concentration of solute particles in the solution, which is then used in the boiling point elevation formula. It ensures that the calculation accounts for the actual number of particles contributing to the colligative property change.
Outlines
🧪 Introduction to Colligative Properties
The video script introduces the concept of colligative properties, focusing on the van 't Hoff factor and its significance in determining the behavior of solutes in a solution. It explains how the van 't Hoff factor accounts for the dissociation of electrolytes into ions, impacting the overall particle concentration in a solution. The script then outlines four major colligative properties: freezing point depression, boiling point elevation, vapor pressure depression, and osmotic pressure. The instructor, Chad, aims to clarify these properties through calculations and common pitfalls, ensuring a better understanding of how solute concentration affects these properties.
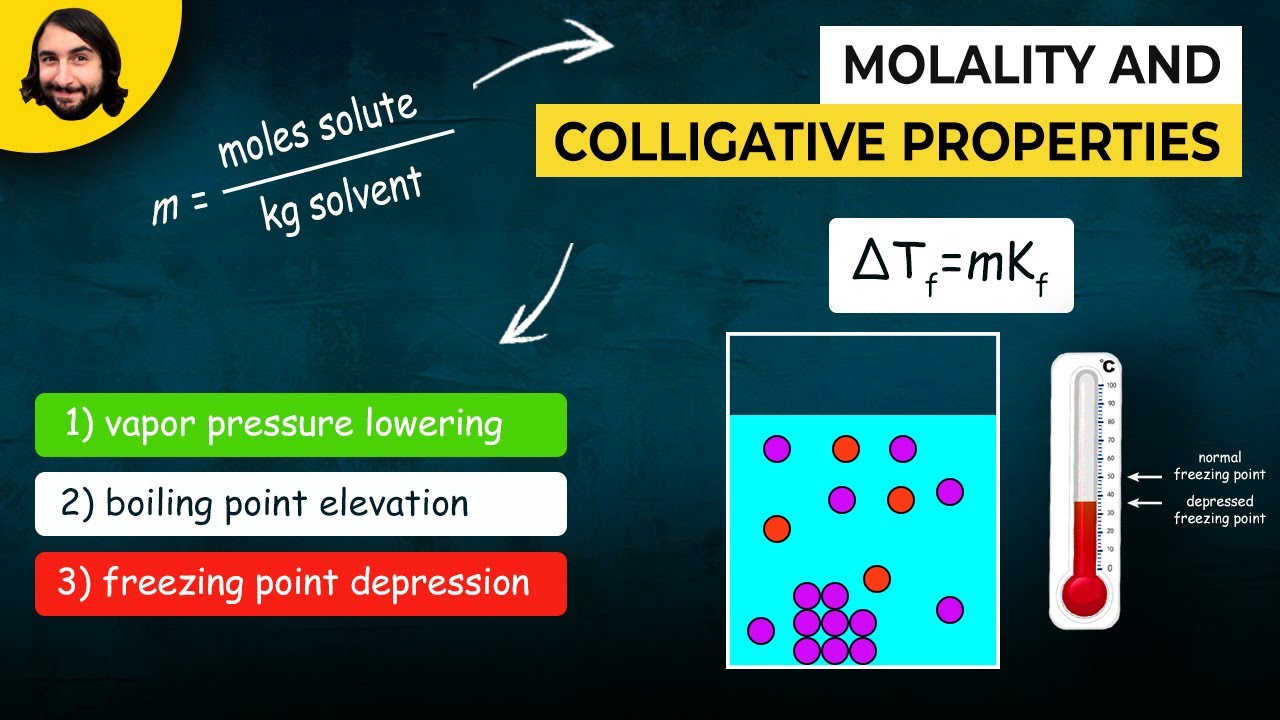
📉 Freezing and Boiling Points in Solutions
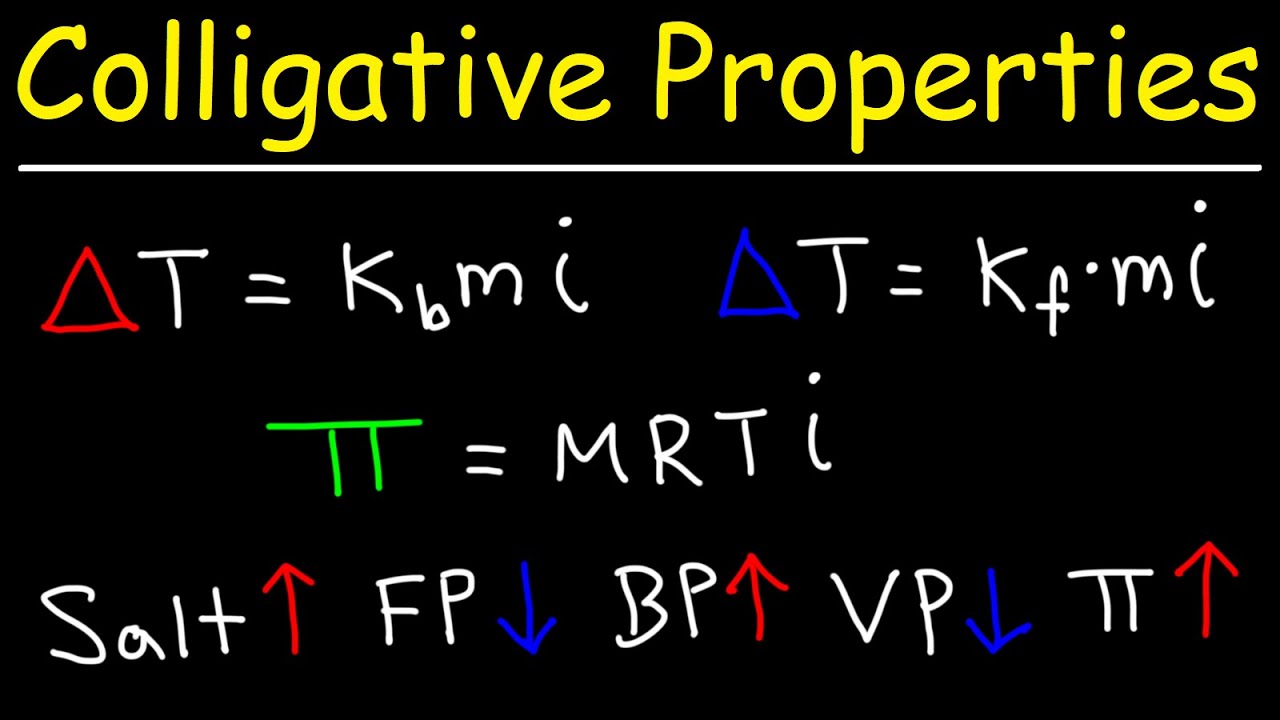
This paragraph delves into the specifics of how solute concentration affects the freezing and boiling points of a solution. It explains that the addition of solute lowers the freezing point and raises the boiling point, with the extent of these changes being related to the number of particles introduced by the solute, as characterized by the van 't Hoff factor. The paragraph also introduces mathematical formulas to calculate these changes, emphasizing the importance of molality and the van 't Hoff factor in determining the overall effect on the solution's phase transition temperatures.
🔍 Impact of Van 't Hoff Factor on Colligative Properties
The script discusses the impact of the van 't Hoff factor on the colligative properties of solutions. It provides examples of different substances, such as NaCl, methanol, barium hydroxide, and aluminum nitrate, to illustrate how the factor influences the concentration of particles in solution. The paragraph clarifies that the van 't Hoff factor is crucial for calculating the extent of changes in colligative properties, especially in the context of electrolytes and non-electrolytes.
📚 Calculations and Conceptual Understanding of Colligative Properties
The paragraph presents a series of calculations and conceptual explanations to further understand the colligative properties. It covers the calculation of freezing points using the freezing point depression formula and the significance of molality and the van 't Hoff factor in these calculations. The script also addresses common misconceptions and emphasizes the importance of understanding why freezing and boiling points change in solutions.
🌡️ Vapor Pressure Depression and Raoult's Law
This section introduces Raoult's Law and its application in determining the vapor pressure depression in solutions. It explains how the presence of a non-volatile solute reduces the vapor pressure above the solution, leading to a depression in vapor pressure. The script provides a step-by-step calculation to determine the vapor pressure of water in a solution containing methanol, using the mole fraction of water and its pure vapor pressure at a given temperature.
💧 Osmotic Pressure and its Relation to Solution Concentration
The final paragraph of the script discusses osmotic pressure, defined as the pressure required to prevent the movement of water across a semi-permeable membrane due to differences in solute concentration. It explains the formula for calculating osmotic pressure and how it relates to the van 't Hoff factor, molarity, and temperature. The script also describes the process of osmosis and how applying pressure greater than the osmotic pressure can reverse the flow of water, a principle utilized in reverse osmosis systems.
Mindmap
Keywords
💡Colligative Properties
💡Van 't Hoff Factor
💡Freezing Point Depression
💡Boiling Point Elevation
💡Raoult's Law
💡Osmotic Pressure
💡Methanol
💡Aluminum Nitrate
💡Solute
💡Molarity
💡Osmosis
Highlights
Introduction to colligative properties and the van 't Hoff factor.
Explanation of how to determine the van 't Hoff factor for compounds.
Discussion on the four major colligative properties: freezing point depression, boiling point elevation, vapor pressure depression, and osmotic pressure.
Calculations for colligative properties and common pitfalls.
The impact of solute concentration on colligative properties.
Differentiation between the van 't Hoff factors of strong electrolytes, non-electrolytes, and polyatomic ions.
How the van 't Hoff factor affects the number of particles in a solution.
Freezing point depression and its relation to the number of solute particles.
Boiling point elevation and its dependence on solute concentration.
Understanding Raoult's Law and its application to vapor pressure depression.
The calculation of freezing point depression using the freezing point constant and molality.
The calculation of boiling point elevation using the boiling point constant and molality.
The concept of osmotic pressure and its relation to molarity, the van 't Hoff factor, and temperature.
Practical applications of osmotic pressure, such as in reverse osmosis systems.
The importance of understanding the principles behind colligative properties for general chemistry.
Transcripts
Browse More Related Video

11.3 Colligative Properties | High School Chemistry
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
Molality and Colligative Properties

Lec-05 I Colligative properties I Applied Chemistry I Chemical Engineering

Molarity , Molality or Mole Fraction || 3D animated explanation || solutions || class12thchemistry

Vapor Pressure - Normal Boiling Point & Clausius Clapeyron Equation
5.0 / 5 (0 votes)
Thanks for rating: