Lec-05 I Colligative properties I Applied Chemistry I Chemical Engineering
TLDRIn this informative lecture, Sugrudi Joshi from LG Institute of Engineering and Technology explores the concept of colligative properties in applied chemistry. The session delves into how solute particles affect a solution's physical properties, such as vapor pressure, boiling point, and freezing point. Key theories like Raoult's Law and the principles behind osmosis and osmotic pressure are explained with clarity, emphasizing their practical applications in areas like RO technology.
Takeaways
- 📚 The lecture is about Applied Chemistry, with the subject code 3130506, and covers the chapter on physical properties and chemical constitution of matter.
- 🔍 In the chapter, the importance of physical properties in understanding molecular structure and matter constitution is discussed.
- 🧪 The preparation of solutions and related terms such as viscosity, optical activity, and magnetic properties are studied.
- 📈 Concepts like molarity, normality, mole fraction, and molality, which are crucial in solution preparation, are introduced.
- 🌡️ Colligative properties are defined as physical properties that depend solely on the concentration of solute particles in a solution.
- 💧 Colligative properties include changes in vapor pressure, freezing point, and boiling point of a solution due to the presence of solute particles.
- 📉 Raoult's Law explains the lowering of vapor pressure in a solution with the addition of a non-volatile solute.
- 🔥 Elevation of boiling point occurs when a non-volatile solute is added to a pure solvent, requiring more heat for the solution to reach its boiling point.
- ❄️ Freezing point depression is the decrease in the freezing point of a solution as the concentration of a non-volatile solute increases.
- 🚰 Osmosis is the process where solvent moves through a semi-permeable membrane from a pure solvent to a solution.
- 🍬 The practical application of osmosis is demonstrated with an example of a sugar solution, highlighting its significance in RO (Reverse Osmosis) technology.
Q & A
What is the subject code for Applied Chemistry in the transcript?
-The subject code for Applied Chemistry is 3130506.
What are the two main components of a solution?
-The two main components of a solution are the solute and the solvent.
What are colligative properties?
-Colligative properties are properties that depend only upon the concentration of the solute particles in a solution.
What are some examples of colligative properties?
-Examples of colligative properties include changes in vapor pressure, freezing point, and boiling point of a solution.
What is Raoult's law and how does it relate to the vapor pressure of a solution?
-Raoult's law states that the vapor pressure of a solution (p) is equal to the product of the vapor pressure of the pure solvent (p) and the mole fraction of the solvent (n/(n+N)), where n is the number of moles of solvent and N is the total number of moles of solute and solvent combined.
How does the addition of a non-volatile solute affect the boiling point of a solvent?
-The addition of a non-volatile solute to a solvent results in an elevation of the boiling point, meaning the boiling point increases.
What is the relationship between the concentration of a solute and the freezing point of a solution?
-As the concentration of a solute increases, the freezing point of the solution decreases, a phenomenon known as freezing point depression.
What is a semi-permeable membrane?
-A semi-permeable membrane is a membrane that allows only the passage of solvent particles and not solute particles.
Define osmosis and explain how it occurs.
-Osmosis is the flow of solvent through a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration. It occurs when solvent particles move from a pure solvent to a solution across the semi-permeable membrane.
What is osmotic pressure and how is it determined?
-Osmotic pressure is the hydrostatic pressure that must be applied to a solution to prevent osmosis of pure solvent into the solution through a semi-permeable membrane. It is determined by the concentration of solute particles in the solution.
How does osmosis play a role in RO (Reverse Osmosis) technology?
-Osmosis is a key principle in RO technology, where an applied pressure greater than the osmotic pressure is used to force solvent particles (usually water) through a semi-permeable membrane, leaving behind the solute particles to produce purified water.
Outlines
📚 Introduction to Applied Chemistry and Colligative Properties
The speaker, Sugrudi Joshi from LG Institute of Engineering and Technology, introduces the topic of Applied Chemistry, specifically focusing on chapter one which discusses the physical properties and chemical constitution of matter. The summary covers the importance of understanding physical properties in relation to molecular structure and constitution. It also delves into the concepts of solution preparation, viscosity, optical activity, and magnetic properties. The speaker then transitions into explaining colligative properties, which depend solely on the concentration of solute particles in a solution. The paragraph concludes with an introduction to Raoult's Law and its role in understanding how solute particles affect vapor pressure.
🌡️ Effects of Solute on Vapor Pressure and Boiling Point
This paragraph discusses the mathematical relationship between the relative lowering of vapor pressure and the addition of non-volatile solute. The speaker explains how the vapor pressure of a solution is affected by the presence of solute, leading to a change in the properties of the solution. The concept of boiling point elevation is introduced, with a detailed explanation of how the addition of a non-volatile solute to a pure solvent results in an increase in boiling point. The speaker uses visual aids to illustrate the process and concludes with the formula that shows the change in boiling point due to solute addition.
❄️ Freezing Point Depression and Osmosis
The speaker continues the discussion on colligative properties by explaining the phenomenon of freezing point depression, where the addition of a non-volatile solute to a pure solvent results in a decrease in the freezing point of the solution. The relationship between the amount of solute and the degree of freezing point depression is explored. The paragraph then introduces osmosis and its three key concepts: semi-permeable membranes, the flow of solvent from pure solvent to solution, and osmotic pressure. The speaker uses diagrams and examples, such as a sugar solution, to illustrate osmosis and its importance in reverse osmosis technology. The summary concludes with a brief mention of the end of chapter one.
Mindmap
Keywords
💡Applied Chemistry
💡Physical Properties
💡Colligative Properties
💡Solute
💡Solvent
💡Raoult's Law
💡Boiling Point Elevation
💡Freezing Point Depression
💡Osmosis
💡Semi-Permeable Membrane
💡Osmotic Pressure
Highlights
Introduction to Applied Chemistry and its subject code 3130506.
Discussion on physical properties and chemical constitution of matter and their importance in understanding molecular structure.
Explanation of terms used in solution preparation, such as viscosity, optical activity, and magnetic properties.
Definition and discussion of colligative properties and their dependence on solute particle concentration.
Colligative properties include changes in vapor pressure, freezing point, and boiling point due to solute addition.
Introduction to Raoult's Law and its role in explaining the lowering of vapor pressure in solutions.
Mathematical formula for relative lowering of vapor pressure and its interpretation.
Explanation of the relationship between mole fraction of solute and relative lowering of vapor pressure.
Elevation of boiling point in solutions with the addition of non-volatile solute and its mathematical representation.
Discussion on freezing point depression and how it continuously decreases with increased solute concentration.
Introduction to osmosis, semi-permeable membranes, and osmotic pressure with definitions and descriptions.
Visual explanation of osmosis through the use of a semi-permeable membrane and its impact on solution levels.
Practical example of osmosis using a sugar solution and its relevance in RO technology.
Summary of chapter number one and its significance in the study of Applied Chemistry.
Transcripts
Browse More Related Video
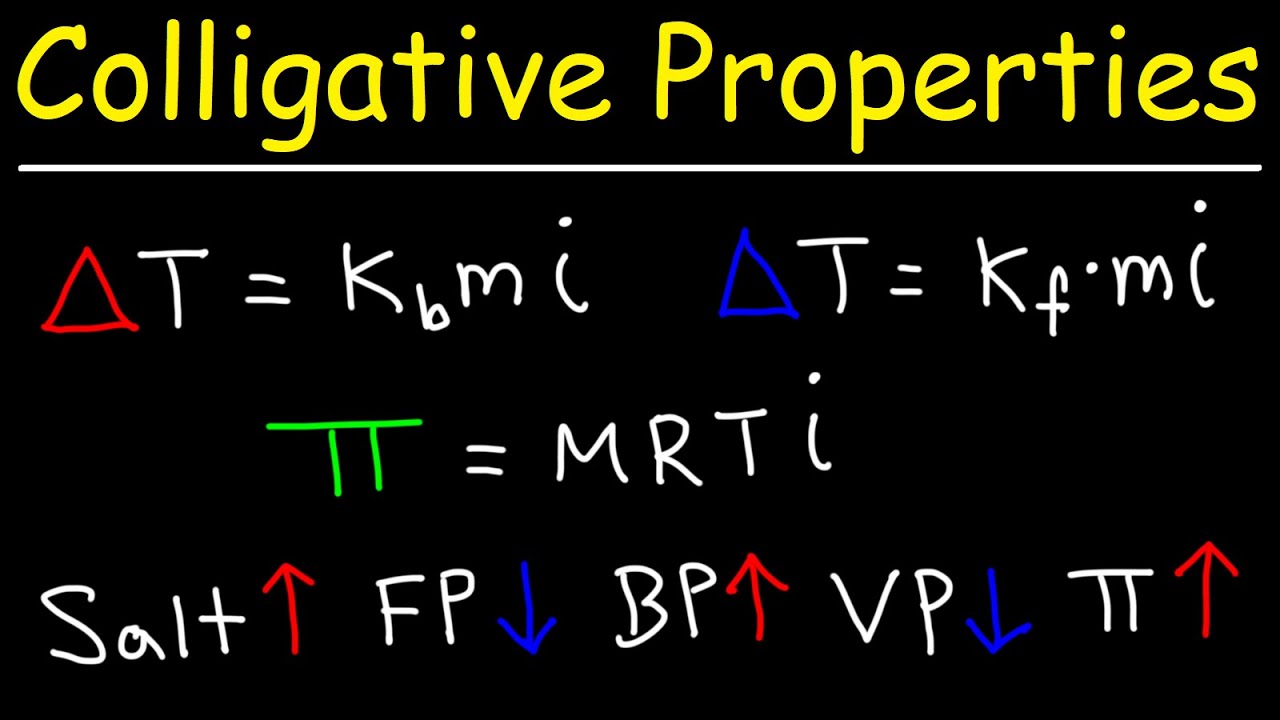
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure
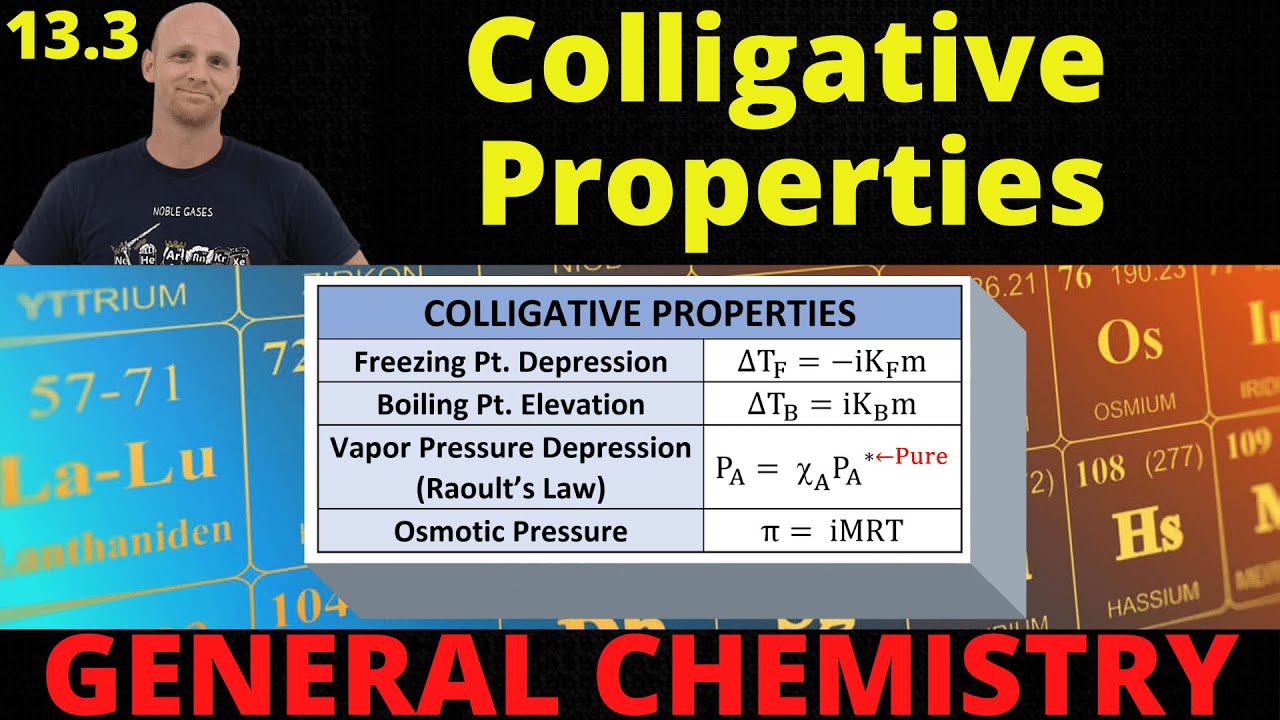
13.3 Colligative Properties | General Chemistry

11.3 Colligative Properties | High School Chemistry
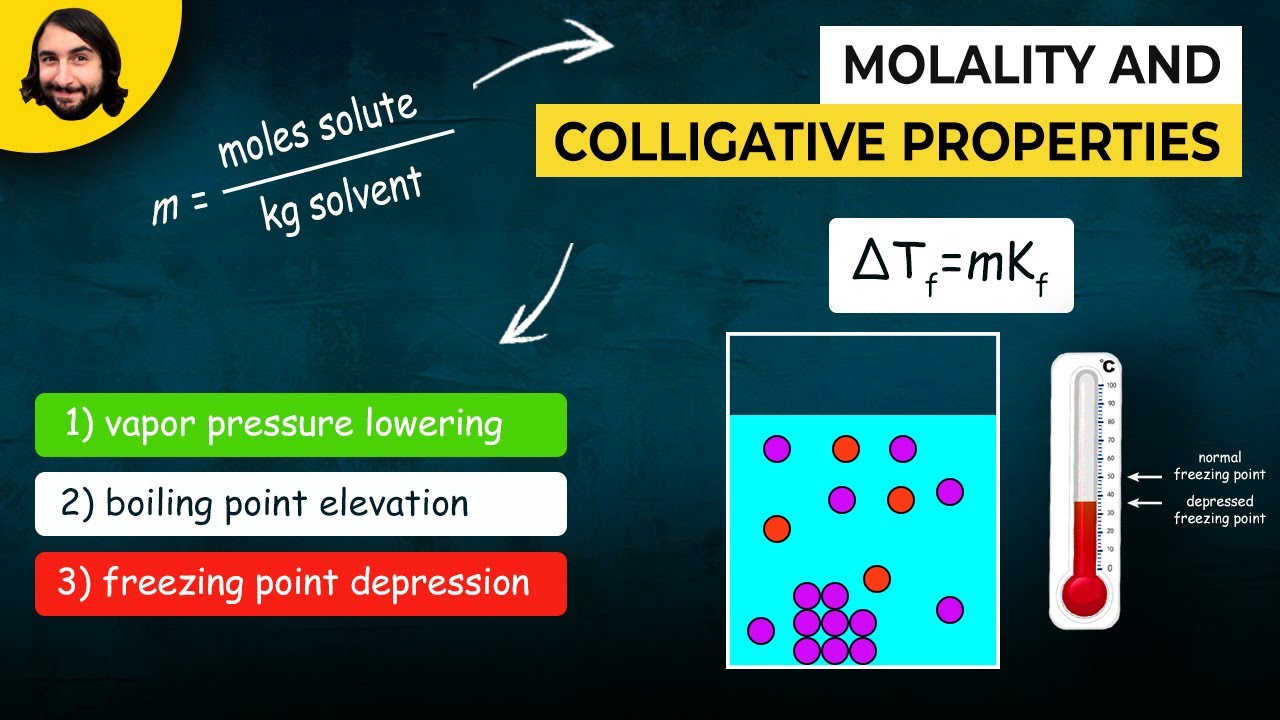
Molality and Colligative Properties

Lec-32 l NMR Spectroscopy l Applied chemistry | Chemical engineering

Lec-37 I Measurement of Turbidity I Applied chemistry I Chemical engineering
5.0 / 5 (0 votes)
Thanks for rating: