Boiling point elevation and freezing point depression | Chemistry | Khan Academy
TLDRThis script delves into the effects of solutes on the boiling and freezing points of solutions, using water as an example. It explains how the introduction of solute particles disrupts the organization of water molecules, making it harder for them to form hydrogen bonds necessary for freezing, thus lowering the freezing point. Conversely, the presence of solutes at the surface reduces the vapor pressure, raising the boiling point. The script also illustrates how the molality and dissociation of solutes, such as sodium chloride versus glucose, influence these temperature changes.
Takeaways
- 🧊 The addition of solute particles to a solvent, such as water, disrupts the regular structure required for freezing, making it more difficult to achieve a solid state.
- 🔥 Solute particles also make it harder for a solution to boil by occupying surface area and reducing the vapor pressure, thus requiring more heat to reach boiling point.
- 💧 The presence of solute particles in a solution increases the disorder, potentially affecting entropy, and makes it more challenging for solvent molecules to organize for phase transitions.
- ❄️ The freezing point of a solution is lowered due to the difficulty in organizing solvent molecules around the solute particles for the formation of hydrogen bonds.
- 🌡 The boiling point of a solution is raised because solute particles at the surface reduce the escape of solvent molecules into the vapor, thus lowering the vapor pressure.
- 📉 The change in boiling or freezing point is proportional to the molality of the solute and the number of particles it dissociates into in the solution.
- 📚 The molality is calculated by the number of moles of solute divided by the kilograms of solvent, and it's crucial for determining the extent of temperature change.
- 🔄 Dissociation of solute molecules, like sodium chloride into ions, doubles the effective molality and therefore the impact on the solution's boiling and freezing points.
- 📉 The lowering of the freezing point is due to the solute's interference with the solvent's ability to form a crystalline structure necessary for solidification.
- 📈 The elevation of the boiling point is a result of the solute's effect on reducing the surface area available for solvent molecules to vaporize, thus increasing the energy needed to reach vapor pressure equilibrium.
- 🔑 The formula for calculating the change in temperature for vaporization involves a constant (k) specific to the solvent and solute, the molality (m), and the dissociation factor (i).
Q & A
What happens when solute particles are added to a liquid like water?
-When solute particles are added to a liquid, they disrupt the regular structure of the liquid molecules, making it harder for them to organize into a crystalline structure necessary for freezing. This results in a lower freezing point and a higher boiling point.
Why does the addition of solute particles make it harder to freeze a liquid?
-The solute particles interfere with the formation of hydrogen bonds between water molecules, which are necessary for the crystalline structure of ice. This makes it more difficult for the water molecules to organize themselves, thus requiring more energy to freeze.
How does the presence of solute particles affect the boiling point of a liquid?
-Solute particles increase the boiling point of a liquid because they occupy surface area, reducing the number of solvent molecules available to vaporize. This results in a lower vapor pressure, requiring more heat to reach the boiling point.
What is the relationship between the molality of a solute and the change in boiling or freezing point of a solution?
-The change in boiling or freezing point is proportional to the molality of the solute multiplied by a constant specific to the solvent and solute. This constant reflects the effect of the solute on the solvent's phase transition temperatures.
Why does the dissociation of solute particles affect the molality and thus the change in boiling or freezing point?
-Dissociation increases the number of particles in the solution, effectively increasing the molality. For example, sodium chloride dissociates into two ions, doubling the molality compared to a non-dissociating solute like glucose, which results in a greater effect on the phase transition temperatures.
What is the role of hydrogen bonds in the freezing process of water?
-Hydrogen bonds are crucial for the formation of the crystalline structure of ice. They help water molecules organize themselves into a regular lattice, which is necessary for freezing. The presence of solute particles can disrupt these bonds, making it harder for water to freeze.
How does the surface area occupied by solute particles influence the vapor pressure and boiling point of a solution?
-Solute particles that occupy the surface area of a liquid reduce the number of solvent molecules that can escape into the vapor phase. This lowers the vapor pressure, making it harder for the liquid to boil at the same temperature as the pure solvent.
What is the significance of the constant k in the equation for calculating the change in boiling or freezing point?
-The constant k represents the sensitivity of the solvent to the presence of solute particles. It is a measure of how much the phase transition temperatures will change per unit of molality of the solute.
How does the molality of a solute affect the phase transition temperatures of a solution?
-The molality of a solute directly influences the phase transition temperatures of a solution. A higher molality results in a greater change in boiling and freezing points, as more solute particles are present to disrupt the solvent's structure and vapor pressure.
What is the difference in the effect of solute particles on the boiling and freezing points of a solution?
-While both boiling and freezing points are affected by the presence of solute particles, the specific effects are different. Solute particles lower the freezing point by disrupting the crystalline structure of the solvent, and they raise the boiling point by reducing the vapor pressure due to surface area occupation.
Outlines
🧊 Effect of Solute on Freezing and Boiling Points
This paragraph discusses how adding a solute to a solvent, such as water, affects its freezing and boiling points. It explains that the introduction of solute particles disrupts the orderly arrangement of water molecules necessary for freezing, making it harder to achieve a crystalline structure and thus lowering the freezing point. The paragraph also explores the concept of vapor pressure and how solute particles at the surface of the liquid reduce the surface area available for solvent molecules to evaporate, leading to a decrease in vapor pressure and an increase in the energy required to reach boiling, thereby raising the boiling point.
🌡️ Calculating Boiling Point Elevation with Molality
This paragraph delves into the quantitative aspect of how solutes affect the boiling point of a solution. It introduces the concept of molality, which is the number of moles of solute per kilogram of solvent, and explains that the change in boiling point is proportional to the product of a constant (k), the molality (m), and the number of particles the solute dissociates into (i). The paragraph provides an example using sodium chloride (NaCl), which dissociates into two ions, doubling its effect on the boiling point. It also contrasts this with glucose, which does not dissociate, thus having a lesser impact on the boiling point.
🧊 Freezing Point Depression and the Role of Solute Dissociation
The final paragraph addresses the impact of solute dissociation on the freezing point of a solution. It emphasizes that the change in freezing point is also proportional to the molality and the number of particles the solute dissociates into. The paragraph illustrates how sodium chloride, which dissociates into two ions, has a greater effect on the freezing point compared to glucose, which remains as a single molecule in solution. This highlights the importance of considering both the molality and the dissociation behavior of solutes when predicting changes in the physical properties of solutions.
Mindmap
Keywords
💡Boiling Point
💡Freezing Point
💡Solute
💡Solvent
💡Molality
💡Hydrogen Bonds
💡Kinetic Energy
💡Vapor Pressure
💡Entropy
💡Disassociation
Highlights
Adding solute to a solution affects its boiling and freezing points.
Water molecules in liquid state are reasonably disorganized but still have hydrogen bonds.
Freezing water involves organizing water molecules into a crystalline structure.
Introduction of solute molecules disrupts the organization of water molecules.
Solute particles make it harder for water molecules to form a regular structure for freezing.
Solute particles lower the melting point of the solution.
Boiling involves water molecules at the surface escaping into vapor.
Solute particles at the surface reduce the vapor pressure, making boiling harder.
Solute particles raise the boiling point of the solution.
The effect of solute on boiling and freezing points is proportional to the molality of the solute.
Molality is calculated by the number of moles of solute per kilogram of solvent.
Different solutes have different constants affecting the boiling and freezing points.
Disassociation of solute molecules affects the molality and thus the effect on boiling and freezing points.
The change in boiling point is calculated by multiplying the constant by the molality and the number of particles the solute disassociates into.
Glucose, which does not disassociate, has a smaller effect on the boiling point compared to sodium chloride.
The change in freezing point is also proportional to the molality of the solute.
Transcripts
Browse More Related Video
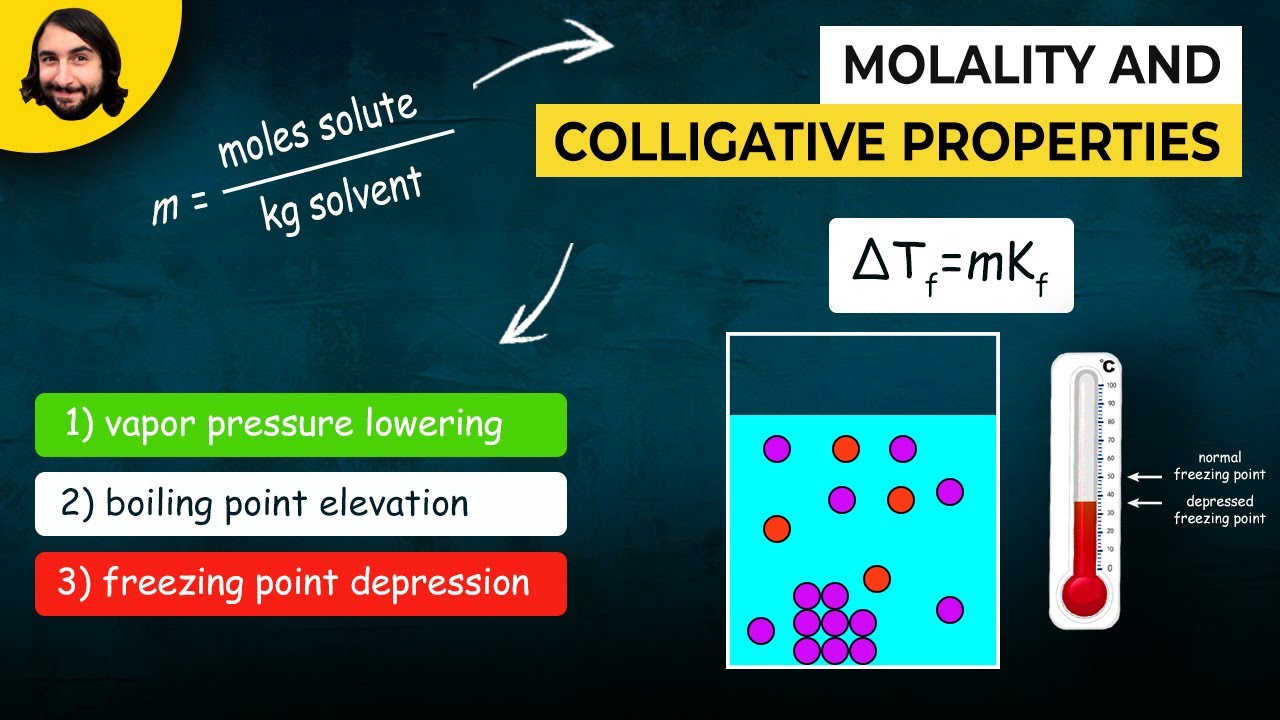
Molality and Colligative Properties
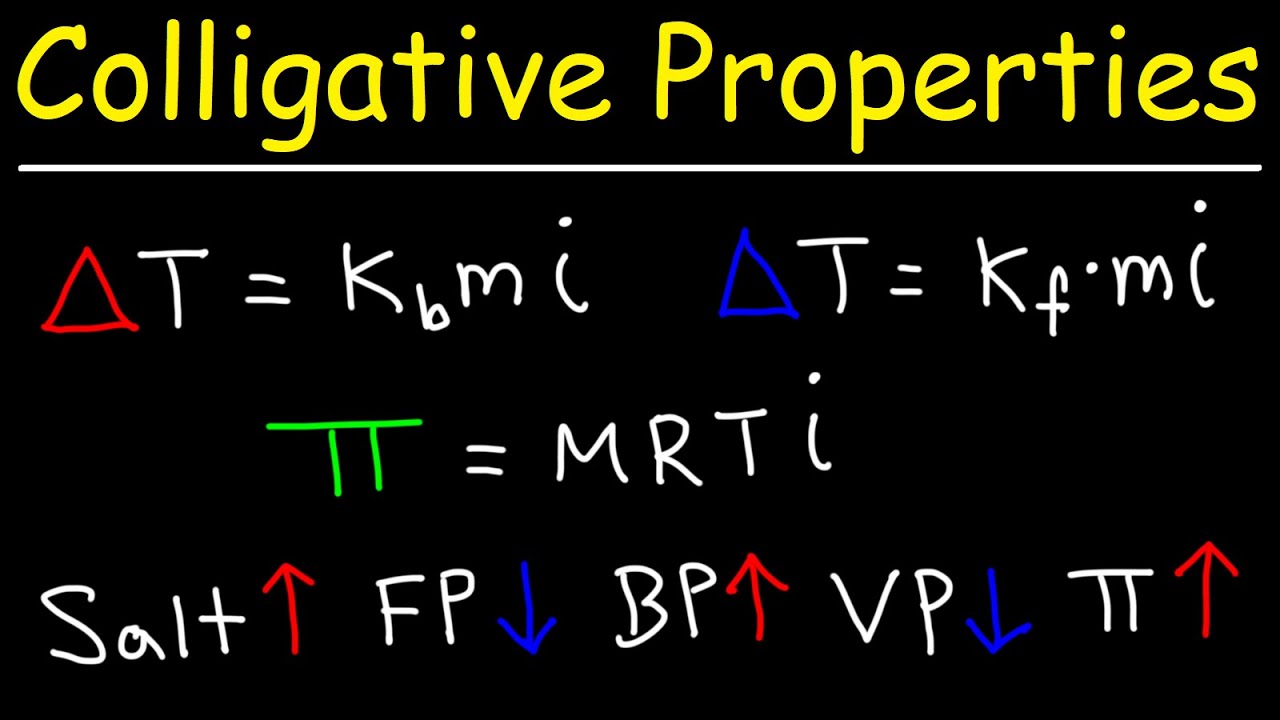
Colligative Properties - Boiling Point Elevation, Freezing Point Depression & Osmotic Pressure

The phase diagram of water

Aqueous Solutions, Dissolving, and Solvation
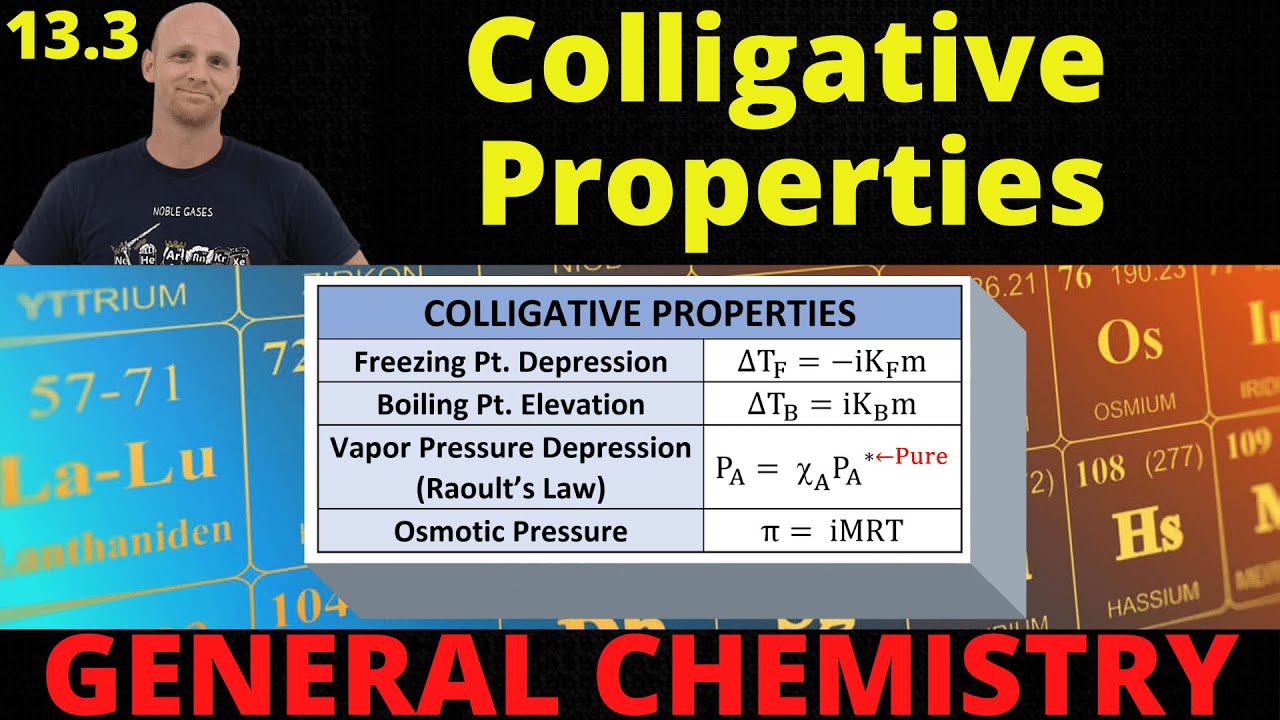
13.3 Colligative Properties | General Chemistry

Vapor Pressure - Normal Boiling Point & Clausius Clapeyron Equation
5.0 / 5 (0 votes)
Thanks for rating: