Chapter 6: Gibbs Free Energy | CHM 214 | 050
TLDRThe video script delves into the concept of Gibbs free energy in thermodynamics, highlighting its role in determining the spontaneity of chemical processes at constant temperature and pressure. It explains that a negative Gibbs free energy (ΔG) indicates a spontaneous process, while a positive ΔG suggests non-spontaneity. However, it clarifies that thermodynamics does not dictate the speed of a process, which is governed by kinetics. The example of carbon allotropes, such as graphite and diamond, illustrates that the lowest Gibbs free energy does not guarantee a rapid transformation, as kinetic barriers may exist.
Takeaways
- 📚 Gibbs free energy (ΔG) is a crucial concept in thermodynamics for chemistry, combining enthalpy (ΔH) and entropy (ΔS) to predict spontaneity of reactions.
- 🔄 ΔG is represented as ΔH - TΔS, where T is the temperature in Kelvin, indicating its reliance on both heat and disorder in a system.
- 🌡️ Gibbs free energy is specific to constant temperature and pressure conditions, much like enthalpy is specific to constant pressure.
- 🚫 A negative ΔG indicates a spontaneous process, meaning it can occur without external intervention, while a positive ΔG signifies a non-spontaneous process.
- ✅ Thermodynamics does not guarantee the occurrence of a process; a negative ΔG is necessary but not sufficient for a reaction to happen on its own.
- 🏃♂️ ΔG does not provide information on the rate of a process, which is determined by kinetics and may involve overcoming energy barriers.
- 💎 The classic example of diamond and graphite illustrates that thermodynamic stability (low Gibbs free energy) does not equate to rapid transformation.
- 🔄 Allotropes such as diamond, graphite, buckyballs, and carbon nanotubes have different arrangements of carbon atoms with varying Gibbs free energies.
- ⏳ Given enough time, higher energy allotropes like diamond will eventually transform into the more stable graphite, though the kinetics are extremely slow.
- 🌟 Diamonds are often considered 'forever' due to their slow kinetics, despite not being the thermodynamically most stable form of carbon.
Q & A
What is Gibbs free energy and how is it represented?
-Gibbs free energy is a thermodynamic potential that combines enthalpy and entropy to determine the spontaneity of a process. It is represented as ΔG, where ΔH is the change in enthalpy and ΔS is the change in entropy of the system, with T being the temperature in Kelvin.
How does Gibbs free energy indicate the spontaneity of a process?
-A negative ΔG value indicates that a process is spontaneous at constant temperature and pressure, meaning it can occur without any external driving force. Conversely, a positive ΔG value suggests that the process is non-spontaneous under the given conditions.
What are the limitations of Gibbs free energy in predicting the occurrence of a process?
-While Gibbs free energy can determine if a process is spontaneous, it does not provide information on the feasibility or the rate at which the process will occur. The rate of a process is determined by kinetic factors and potential energy barriers.
What is the relationship between Gibbs free energy, enthalpy, and entropy?
-Gibbs free energy (ΔG) is defined as the change in enthalpy (ΔH) minus the product of the temperature (T) and the change in entropy (ΔS). This relationship shows how the heat content (enthalpy) and disorder (entropy) of a system contribute to its overall spontaneity.
What is the significance of the temperature factor in Gibbs free energy?
-The temperature (T) in Gibbs free energy is crucial because it affects the entropy term (ΔS). A higher temperature can increase the entropy change, which can influence whether a process is spontaneous or not.
Can a process with a positive ΔG ever occur spontaneously?
-A process with a positive ΔG is generally non-spontaneous under the specified conditions. However, it can occur spontaneously if the conditions are changed, such as by altering the temperature or pressure, or if external work is applied to the system.
What is an example of a process that is spontaneous but has a slow rate?
-The transformation of diamond into graphite is an example of a spontaneous process with a slow rate. Although graphite has a lower Gibbs free energy and is more stable, the kinetics of rearranging the carbon atoms in diamond to form graphite are very slow, making diamonds persistent in their structure over time.
What is the difference between spontaneity and feasibility in thermodynamics?
-Spontaneity refers to the natural tendency of a process to occur without external influence, as indicated by a negative ΔG. Feasibility, on the other hand, is about whether a process can physically happen given the conditions and potential energy barriers, which may not be directly related to the thermodynamic spontaneity.
Why is Gibbs free energy important in chemical reactions?
-Gibbs free energy is important in chemical reactions because it provides a quantitative measure of whether a reaction will proceed spontaneously at constant temperature and pressure. It helps in predicting the direction of chemical reactions and the conditions under which they will occur.
How does the Gibbs free energy relate to the second law of thermodynamics?
-The second law of thermodynamics states that in an isolated system, the total entropy will increase over time. Gibbs free energy is related to this law as it considers the entropy change (ΔS) in its calculation, thus reflecting the natural tendency towards increased disorder or entropy.
What is the role of kinetics in determining the rate of a spontaneous process?
-Kinetics plays a crucial role in determining the rate at which a spontaneous process occurs. Even if a process is thermodynamically favorable (negative ΔG), kinetic factors such as activation energy barriers can slow down the reaction rate, potentially preventing it from happening at a measurable pace.
Outlines
🌡️ Gibbs Free Energy in Thermodynamics
This paragraph introduces Gibbs free energy as a crucial concept in thermodynamics, particularly in chemistry. It explains that Gibbs free energy, represented as delta G, combines enthalpy (delta H) and entropy (delta S) to predict the spontaneity of a chemical reaction. The balance of heat and disorder is used to determine whether a process will occur without external influence at constant temperature and pressure. A negative delta G indicates a spontaneous process, while a positive delta G suggests the process is non-spontaneous. However, it's emphasized that thermodynamics does not provide information on the rate of a process, which is governed by kinetics. The example of carbon allotropes, such as graphite and diamond, illustrates that the lowest Gibbs free energy does not necessarily dictate the stability or transformation rate of a substance.
Mindmap
Keywords
💡Thermodynamics
💡Gibbs Free Energy
💡Enthalpy
💡Entropy
💡Spontaneous Process
💡Constant Temperature and Pressure
💡Allotropes
💡Kinetics
💡Graphite
💡Diamond
💡Buckyballs
Highlights
Gibbs free energy is a crucial variable in thermodynamics for chemistry.
Gibbs free energy combines enthalpy and entropy to determine spontaneity of reactions.
The formula for Gibbs free energy is represented as delta G = delta H - T delta S.
Enthalpy (delta H) refers to the heat involved in a process at constant pressure.
Entropy (delta S) is a measure of disorder in a system.
Temperature (T) is a factor in the calculation of Gibbs free energy.
A negative Gibbs free energy indicates a spontaneous process at constant temperature and pressure.
A positive Gibbs free energy signifies a non-spontaneous process.
Thermodynamics does not provide information on the rate of a process; this is determined by kinetics.
Gibbs free energy does not dictate the possibility of a process happening, only its spontaneity.
The transformation of diamond to graphite, although thermodynamically favorable, is slow due to kinetic barriers.
Graphite is the lowest Gibbs free energy allotrope of carbon.
Other carbon allotropes like buckyballs and carbon nanotubes are stable but higher in energy.
Kinetics play a crucial role in the actual occurrence and speed of a process, not just its spontaneity.
The classic phrase 'diamonds are forever' is not entirely accurate from a thermodynamic standpoint.
Understanding Gibbs free energy is vital for predicting the spontaneity of chemical reactions.
The relationship between enthalpy, entropy, and temperature is fundamental in assessing process spontaneity.
The study of Gibbs free energy bridges thermodynamics and kinetics in understanding process dynamics.
Transcripts
Browse More Related Video

Gibbs free energy and spontaneous reactions | Biology | Khan Academy
Chapter 6: K and Standard Gibbs Energy | CHM 214 | 051
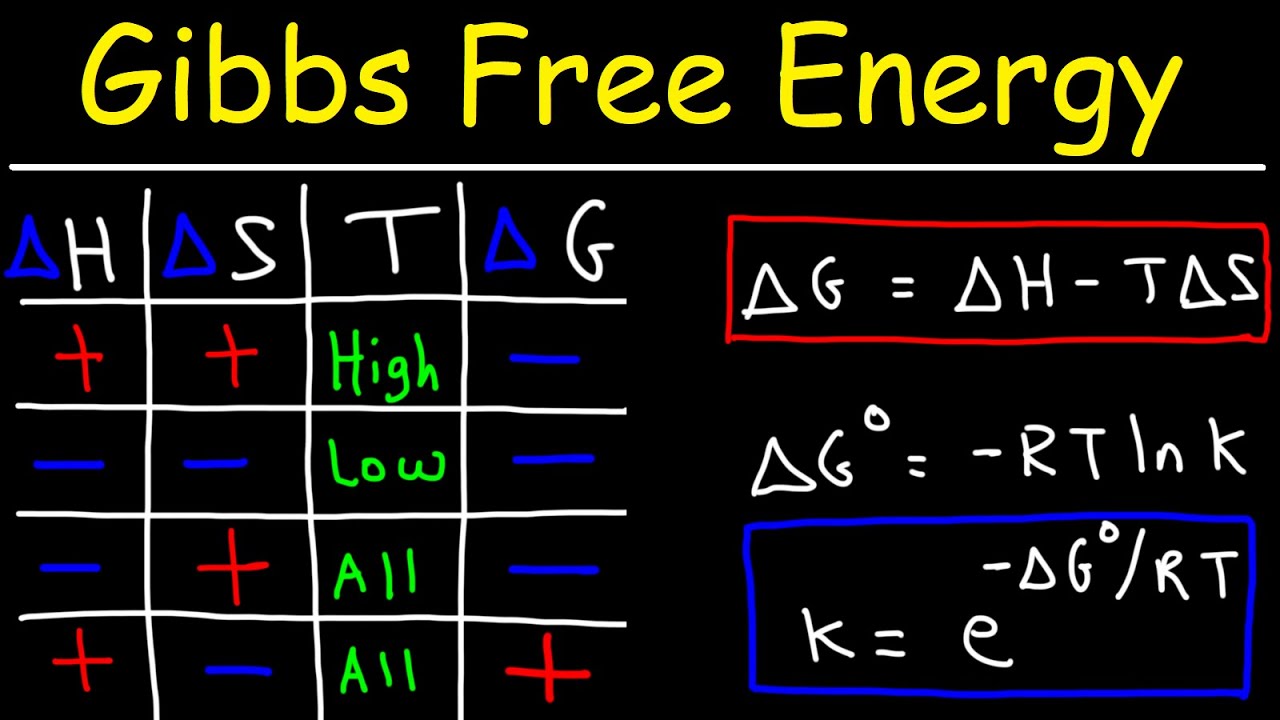
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry
[H2 Chemistry] 2021 Topic 5 Energetics 3

6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: