6. Hydrogen Atom Wavefunctions (Orbitals)
TLDRThe transcript discusses the significance of quantum numbers in describing electron behavior in atomic orbitals, focusing on the hydrogen atom. It explains the concepts of binding energies, wave functions, and orbitals, highlighting the spherical symmetry of s orbitals and the lobed shape of p orbitals. The lecture also touches on the implications of these properties for technologies like nano MRI and emphasizes the importance of understanding electron distribution and shielding effects for grasping chemical properties and reactions.
Takeaways
- 📚 The Schrodinger equation provides binding energies and wave functions for the hydrogen atom, which can be described using quantum numbers.
- 🔢 Quantum numbers include the principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (m), and spin quantum number (m_s).
- 🌐 Wave functions, or orbitals, are represented by the quantum numbers n, l, and m, and can be visualized using different plots to understand electron probability distributions.
- 📈 The radial probability distribution plot shows the likelihood of finding an electron at a certain distance from the nucleus, with the most probable distance indicated.
- 🌟 S orbitals are spherically symmetrical, meaning their probability density depends only on the radius and not on the angles.
- 💫 P orbitals have a different shape with two lobes separated by a nodal plane, indicating a dependence on angles and a lack of electron probability at the nucleus.
- 📊 The number of radial nodes in an orbital can be calculated using the formula n - 1 - l, which represents values of r for which the wave function squared (probability density) is zero.
- 🔄 The angular nodes or nodal planes in p orbitals arise from the angular component of the wave function and are values of theta and phi where the wave function squared is zero.
- 🔧 The size of an orbital, or the most probable radius for finding an electron, increases with the principal quantum number (n) and decreases with the angular momentum quantum number (l).
- 🔋 Electrons in s states are the least shielded because they have a higher probability of being close to the nucleus, which has implications for energy levels and chemical properties.
- 🚫 Pauli's exclusion principle states that no two electrons can have the same set of four quantum numbers, meaning that an orbital can hold a maximum of two electrons with opposite spins.
Q & A
What is the significance of the Schrodinger equation in the context of the hydrogen atom and binding energies?
-The Schrodinger equation is crucial for determining the binding energies and wave functions of the hydrogen atom. It provides information about the quantized energy levels and the orbitals, which are essential for understanding the atom's structure and behavior.
What are the three quantum numbers that describe an orbital, and how are they related to the shape of the orbital?
-The three quantum numbers that describe an orbital are n (principal quantum number), l (angular momentum quantum number), and m (magnetic quantum number). The value of l determines the subshell (s, p, d, f) and the shape of the orbital, while m specifies the orientation of the orbital in space.
How does the value of n relate to the energy level or shell of an atom?
-The value of n (principal quantum number) directly corresponds to the energy level or shell of an atom. As n increases, the energy level increases, and the electron is further away from the nucleus.
What is the significance of the Rydberg constant in the context of binding energies?
-The Rydberg constant (RH) is used in the formula for the binding energy of an electron in the hydrogen atom. The binding energy is given by minus the Rydberg constant divided by n squared, where n is the principal quantum number.
How does the concept of degenerate orbitals apply to the hydrogen atom?
-In the hydrogen atom, orbitals with the same n value are degenerate, meaning they have the same energy. This is because the energy levels only depend on the principal quantum number n and not on l or m for a single-electron system like hydrogen.
What is the role of hydrogen in imaging technologies like MRI, as described by Benjamin Ofori-Okai?
-Hydrogen, due to its abundance in water and other biological molecules, is fundamental in MRI technology. The magnetic signal generated by hydrogen atoms is used to create detailed images of organs and tissues within the human body. In nano MRI, the focus is on imaging at a much smaller scale, such as viruses and individual cells.
What is the importance of the wave function in understanding the behavior of electrons around the nucleus?
-The wave function provides information about the probability density of finding an electron at a certain location around the nucleus. It helps to describe the shape of the orbital and the likelihood of an electron being present in a particular region of space.
How does the angular wave function differ for s and p orbitals?
-For s orbitals, the angular wave function is a constant, meaning that the probability of finding an electron is independent of the angles theta and phi, resulting in spherical symmetry. In contrast, p orbitals have a non-constant angular wave function, which introduces a dependence on angles, leading to an asymmetric shape with nodal planes where the probability is zero.
What is a radial node in the context of electron orbitals?
-A radial node is a value of r (radius) for which the wave function, or the probability density (wave function squared), is zero. It represents a region in space where there is no probability of finding the electron.
How can the number of radial nodes be calculated for a given orbital?
-The number of radial nodes can be calculated using the formula n minus 1 minus l, where n is the principal quantum number and l is the angular momentum quantum number.
What is the fourth quantum number, and how does it relate to the Pauli exclusion principle?
-The fourth quantum number is the spin quantum number (m_sub_s), which can have values of plus or minus 1/2. It relates to the Pauli exclusion principle by stating that no two electrons in an atom can have the same set of four quantum numbers, which means that an orbital can hold a maximum of two electrons with opposite spins.
Outlines
📚 Introduction to Quantum Numbers and Orbitals
The paragraph introduces the concept of quantum numbers and orbitals, specifically focusing on the hydrogen atom. It explains the Schrodinger equation and how it provides information about wave functions and quantum numbers. The three quantum numbers (n, l, m) are described, with n representing the energy level or shell, l the angular momentum quantum number related to the subshell or shape of the orbital, and m the magnetic quantum number that describes the behavior in a magnetic field and orientation of the orbital. The importance of these numbers in describing an electron's behavior and its probability of being found in a certain region around the nucleus is emphasized.
🌟 Understanding Orbital Nomenclature and Energy Levels
This paragraph delves into the terminology used by chemists to describe orbitals, such as the use of s, p, d, f for different subshells. It explains how the quantum numbers n, l, and m are related to the wave function and the specific orbital, with examples like 1s, 2s, and 2p orbitals. The concept of degenerate orbitals within the same energy level for a hydrogen atom is introduced, along with the binding energy calculation for electrons in these orbitals. The paragraph also touches on the significance of hydrogen in imaging technologies like MRI and the importance of understanding electron behavior in atoms.
🧪 Nanoscale Magnetic Resonance Imaging (nano MRI)
A graduate student named Benjamin Ofori-Okai discusses his work on nanoscale magnetic resonance imaging (nano MRI), a technique that aims to perform imaging on a much smaller scale than traditional MRI. The technology involves a probe sensitive to local magnetic fields and uses the properties of hydrogen atoms to generate images. The potential applications of this technology include observing viruses in real-time and understanding their interaction with cells, which could lead to advancements in medical imaging and treatment of diseases.
📈 Probability Density and Electron Distribution
The paragraph focuses on the significance of the wave function in understanding the probability density and the distribution of electrons around the nucleus. It explains how the wave function depends on the radius and two angles (theta and phi), and how this relates to the likelihood of finding an electron in a specific location.
Mindmap
Keywords
💡Quantum Numbers
💡Schrodinger Equation
💡Wave Functions
💡Orbitals
💡Binding Energy
💡Angular Momentum
💡Electron Spin
💡Pauli Exclusion Principle
💡Radial Probability Distribution
💡Nodal Planes
Highlights
The lecture discusses the importance of understanding wave functions and quantum numbers in relation to the behavior of electrons in atoms, particularly focusing on the hydrogen atom.
Three quantum numbers are necessary to describe an orbital: n (principal quantum number), l (angular momentum quantum number), and m (magnetic quantum number).
The Schrodinger equation provides information about binding energies and wave functions, which are crucial for understanding atomic structure.
Spherical symmetry is a key characteristic of s orbitals, meaning the probability of finding an electron depends solely on the radius and not on the angles.
P orbitals are not spherically symmetric due to their dependence on angular components, leading to a shape consisting of two lobes separated by a nodal plane.
The number of radial nodes in an orbital can be calculated using the formula n - 1 - l, which is significant for understanding the distribution of electrons.
The concept of degenerate orbitals is introduced, explaining that for a hydrogen atom, orbitals with the same n value have the same energy.
The lecture touches on the application of nanoscale magnetic resonance imaging (nano MRI), showing the practical implications of understanding atomic-level electron behavior.
The significance of hydrogen in various imaging techniques is highlighted, emphasizing its importance in both theoretical and applied sciences.
The wave function's probability density is central to understanding the likelihood of finding an electron at a specific location in space.
The radial and angular components of the wave function are detailed, explaining how they contribute to the overall shape and characteristics of orbitals.
The concept of nodes in orbitals is explained, with radial nodes representing regions of zero probability density for electron presence.
The relationship between the size of an orbital and the shielding of electrons is discussed, revealing counterintuitive trends based on the value of n and l.
Pauli's exclusion principle is introduced, stating that no two electrons can have the same set of four quantum numbers, leading to the conclusion that an orbital can hold a maximum of two electrons.
The lecture concludes with a discussion on the fourth quantum number, m_sub_s, which describes electron spin and is crucial for a complete description of an electron's state.
Transcripts
Browse More Related Video

Quantum Numbers, Atomic Orbitals, and Electron Configurations

Lecture 13 - Quantum numbers
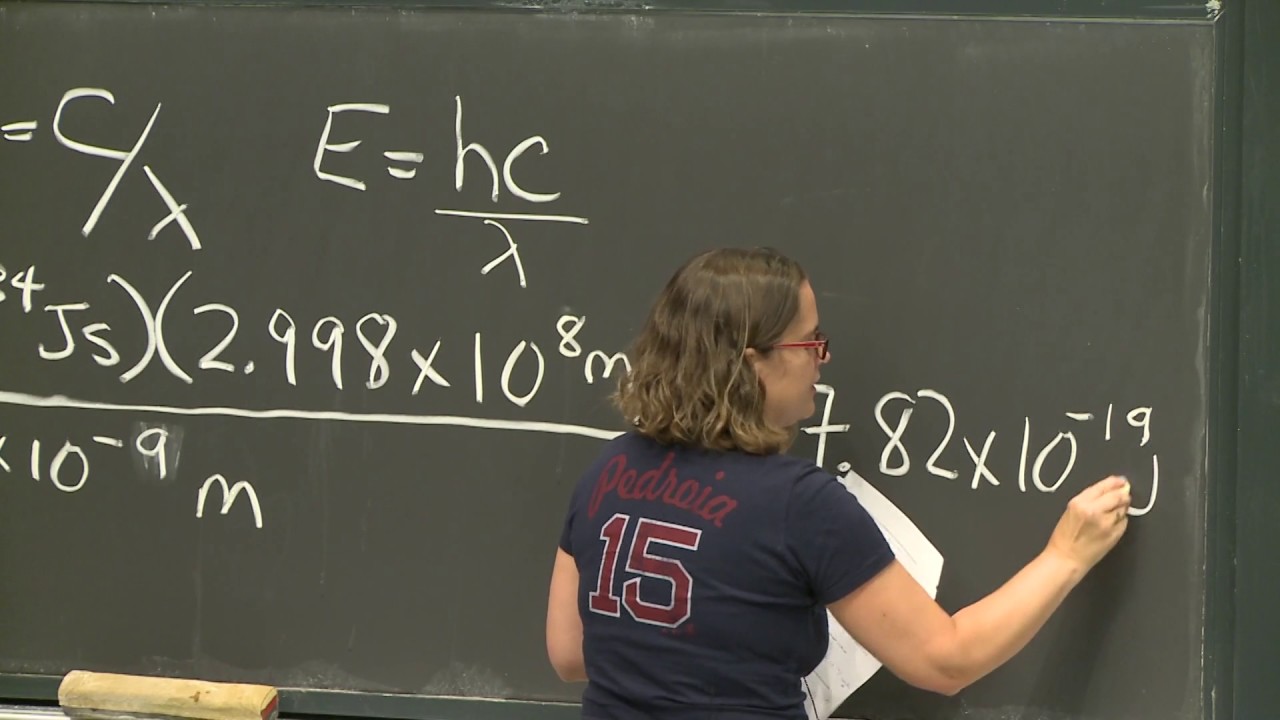
4. Wave-Particle Duality of Matter; Schrödinger Equation

SPDF orbitals Explained - 4 Quantum Numbers, Electron Configuration, & Orbital Diagrams

Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy

Atomic Structure full topic
5.0 / 5 (0 votes)
Thanks for rating: