4. Wave-Particle Duality of Matter; Schrödinger Equation
TLDRThis script from an MIT OpenCourseWare lecture delves into the quantum mechanics underlying the photoelectric effect, exploring light's dual particle-wave nature and its implications for understanding electron behavior. It introduces the concept of photons having momentum and matter exhibiting wave properties, as demonstrated by de Broglie's wavelength formula. The lecture also touches on the significance of quantum mechanics in modern applications, such as quantum dots in medical imaging, and the ongoing relevance of the Schrodinger equation in explaining atomic stability and predicting electron orbitals.
Takeaways
- 📚 The lecture discusses the photoelectric effect, emphasizing the particle nature of light, and how it was instrumental in understanding quantized energy of particles.
- 🌟 It explains the concept of photons having momentum, derived from the photoelectric effect and further supported by experiments like those conducted by Arthur Compton.
- 🌌 The wave-particle duality of light is contrasted with the emerging concept of matter waves proposed by de Broglie, suggesting that particles like electrons also exhibit wave-like properties.
- 🔬 An in-class demonstration of the photoelectric effect is described, predicting the ejection of electrons from a zinc surface when illuminated by light of sufficient energy.
- 🔢 The script includes calculations to determine the energy of photons emitted by different light sources and their ability to eject electrons based on the work function of zinc.
- 🚀 The importance of quantum mechanics and the Schrödinger equation in understanding the behavior of subatomic particles is highlighted, especially when their wavelengths are comparable to their size.
- 🧬 Current research applications of quantum mechanics, such as in the development and understanding of quantum dots for medical applications like tumor removal, are mentioned.
- 🤔 The script ponders the philosophical question of why certain scientific breakthroughs, like the Schrödinger equation, were made by certain individuals and not others.
- 💡 The Schrödinger equation is presented as a fundamental tool in quantum mechanics that, while complex, provides insights into the binding energies and orbitals of electrons.
- 🔍 The lecture touches on the ongoing research in quantum mechanics, including its application to large systems like proteins and materials for energy solutions.
- 📈 The significance of the Rydberg constant in simplifying the Schrödinger equation for the hydrogen atom and its relation to the principal quantum number is explained.
Q & A
What is the photoelectric effect and why is it significant in understanding the properties of light?
-The photoelectric effect is a phenomenon where light ejects electrons from a material. It's significant because it demonstrated that light has particle-like properties, with quantized energy, which was crucial in the development of quantum mechanics.
What is the relationship between the energy of incoming photons and the work function for an electron to be ejected?
-The energy of an incoming photon must be equal to or greater than the work function (threshold energy) of the material for an electron to be ejected. If the photon's energy is less than the work function, the electron will not be ejected.
Why can't the energy of multiple photons with less than the threshold energy each add up to eject an electron?
-The energy of photons cannot be added if each photon has less than the threshold energy because, similar to needing a certain amount of knowledge to pass a test, each photon must individually have enough energy to overcome the work function. The process is quantized and cannot be accumulated from multiple insufficient interactions.
How is the energy of a photon related to its wavelength?
-The energy of a photon is inversely related to its wavelength. This relationship is given by the equation E = (hc) / λ, where E is the energy, h is Planck's constant, c is the speed of light, and λ is the wavelength of the photon.
What is the significance of the de Broglie wavelength and how does it relate to the momentum of particles?
-The de Broglie wavelength is a concept that extends the wave-particle duality to matter, suggesting that all particles have wave-like properties. The wavelength is given by λ = h / (mv), where h is Planck's constant, m is the mass of the particle, and v is its velocity. This relationship shows that the momentum of a particle is inversely related to its wavelength.
Why don't we observe the wave-like behavior of macroscopic objects like a baseball?
-The de Broglie wavelength of macroscopic objects like a baseball is extremely small and undetectable due to their large mass and typical velocities, making their wave-like properties negligible and unobservable.
What is the Schrodinger equation and why is it important in quantum mechanics?
-The Schrodinger equation is a fundamental equation in quantum mechanics that describes the wave-like behavior of particles and their interactions with energy. It is important because it provides a way to calculate the quantized energy levels of systems and predict the behavior of particles, such as electrons in atoms.
What is the significance of the principle quantum number (n) in the context of the Schrodinger equation for the hydrogen atom?
-The principle quantum number (n) is a positive integer that comes from the solutions to the Schrodinger equation for the hydrogen atom. It determines the energy levels of the electron and is directly related to the size and shape of the electron's orbital.
How does the Schrodinger equation explain the stability of the hydrogen atom?
-The Schrodinger equation allows for the calculation of the binding energy of the electron to the nucleus in a hydrogen atom. It shows that the energy levels are quantized and that the electron occupies specific orbitals, preventing it from crashing into the nucleus, thus explaining the atom's stability.
What is the Rydberg constant and how does it simplify the equation for the binding energy of the hydrogen atom?
-The Rydberg constant (RH) is a constant that combines several constants from the full equation for the binding energy of the hydrogen atom. It simplifies the equation to E = -RH / n^2, making it easier to calculate the energy levels for different values of the principle quantum number (n).
Why is quantum mechanics still an active area of research, even though the Schrodinger equation was developed many years ago?
-Quantum mechanics is still an active area of research because it has applications in many modern technologies and materials science. Improving algorithms and computational methods allows scientists to apply quantum mechanics to larger and more complex systems, such as proteins and materials for new batteries and electrodes.
Outlines
📚 Introduction to MIT OpenCourseWare and the Photoelectric Effect
The script begins with an introduction to MIT OpenCourseWare, a free educational resource supported by donations, and then transitions into a classroom setting where Professor Catherine Drennan is discussing the photoelectric effect. The photoelectric effect is a phenomenon where light ejects electrons from a material, and it is explained through the concept of photons having energy. The class explores the conditions under which electrons are ejected, such as the energy of incoming photons needing to be equal to or greater than the work function of the material. The discussion includes a practical example of adding photons with insufficient energy and emphasizes that this does not result in electron ejection, akin to individual knowledge required to pass an exam.
🔬 Exploring Photons, Electrons, and the Photoelectric Effect
This paragraph delves deeper into the photoelectric effect, discussing the relationship between photons, their energy, and the ejection of electrons from a metal surface. It introduces the concept that each photon must have enough energy to meet the threshold required to eject an electron. The script mentions an in-class demonstration using a zinc surface and different light sources with varying wavelengths to predict and observe electron ejection. The paragraph also covers the terminology and concepts relevant to solving problems related to the photoelectric effect, such as the energy of photons, the kinetic energy of electrons, and the importance of understanding units like eV and joules.
🧪 In-Class Demonstration of the Photoelectric Effect
The script describes an in-class experiment aimed at demonstrating the photoelectric effect. The experiment involves shining different light sources with varying wavelengths onto a zinc surface to see if they can eject electrons. The calculations for the energy of photons emitted by a UV lamp and a red laser pointer are detailed, with the UV light found to have sufficient energy to eject electrons while the red light does not. The experiment also includes a calculation of the number of photons emitted by a laser in 60 seconds, given an intensity of one milliwatt, resulting in a large number of photons.
🌟 Light as Both a Wave and a Particle
This section of the script discusses the dual nature of light, which exhibits properties of both a wave and a particle. It explains how the photoelectric effect provided evidence for light's particle-like properties, with quantized energy in the form of photons. The script also touches on the concept of photons having momentum, as proposed by Einstein, and the related experiments by Arthur Compton. The discussion then shifts to the wave-like properties of matter, introduced by de Broglie, who suggested that particles like electrons could also exhibit wave-like behavior, with wavelengths calculated based on their momentum.
🏞️ The Wavelength of Everyday Objects and Electrons
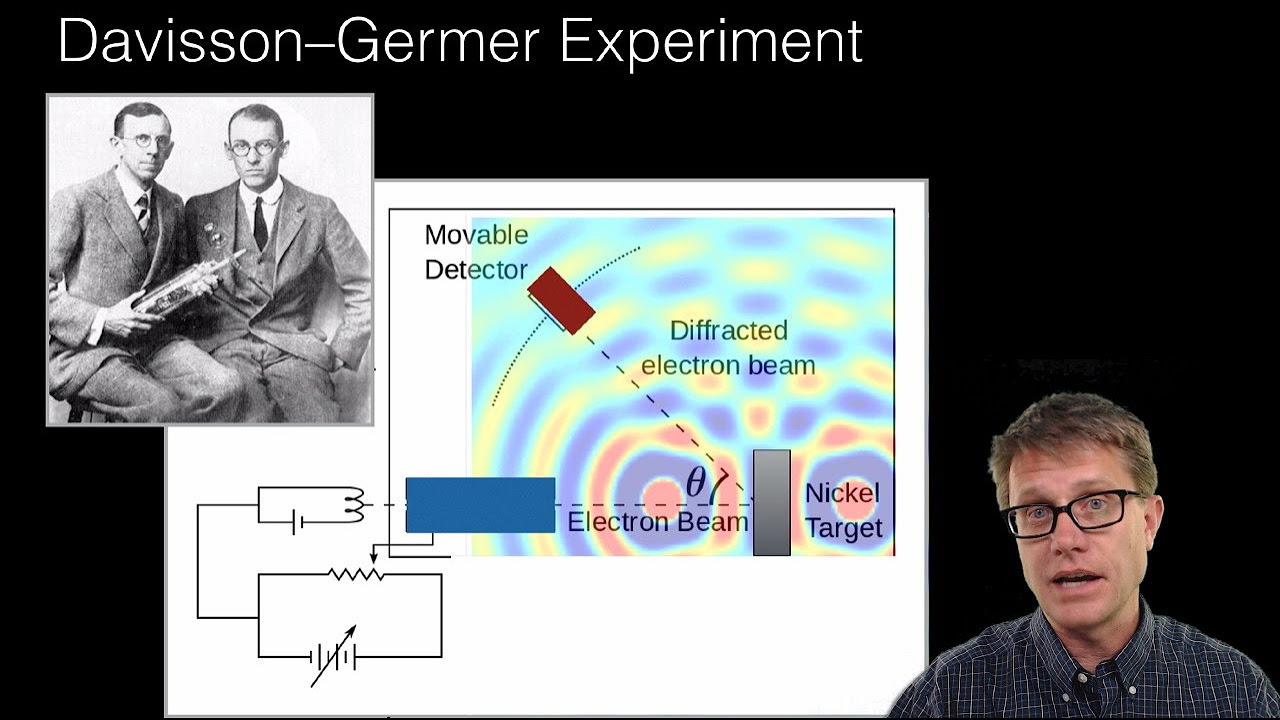
The script explores the wave-like properties of matter further by comparing the calculated wavelengths of everyday objects, such as a baseball, with that of an electron. It explains that while the wavelength of a baseball is too small to detect, the wavelength of an electron is significant when compared to the size of atoms. This makes the wave-like properties of electrons observable and important in the context of atomic and subatomic structures. The paragraph also discusses the observation of diffraction patterns produced by electrons, which is a wave-like property, and the historical significance of this discovery by scientists like JP Thomson.
🌱 Quantum Mechanics and Its Relevance Today
This part of the script discusses the importance of quantum mechanics in modern research and applications. It highlights the unique properties of small particles, such as quantum dots, which have discrete energy levels and emit light of specific energies when electrons transition between these levels. The script mentions ongoing research in the Bawendi Lab at MIT, where quantum dots are being developed for medical applications, such as tumor identification during surgery. The potential of quantum mechanics in developing new materials, batteries, and electrodes for solving energy problems is also emphasized.
🧬 The Schrodinger Equation and Quantum Mechanics
The script introduces the Schrodinger equation, a fundamental component of quantum mechanics, which is likened to Newton's equations in classical mechanics. The equation is presented in its simplest form, and its purpose in describing the wave-like properties of particles, such as electrons, is explained. The paragraph discusses the challenges of solving the equation due to the three-dimensional and dynamic nature of electron behavior in atoms. It also touches on the historical context of the development of quantum mechanics and the Schrodinger equation's role in understanding the stability of the hydrogen atom.
🔬 Current Research and Applications of Quantum Mechanics
This section of the script highlights ongoing research in quantum mechanics, focusing on the work of Professor Heather Kulik, who is developing computational methods to analyze large systems like proteins using quantum mechanics. The importance of such research is underscored by its potential applications in solving energy problems and understanding materials for new batteries and electrodes. The script also mentions the upcoming demonstration of the hydrogen atom spectrum to illustrate the predictive power of the Schrodinger equation in explaining binding energies.
🎓 Upcoming Demonstration and Student Engagement
The final paragraph of the script invites students to an upcoming demonstration and clicker competition, which will further explore the concepts of quantum mechanics and the Schrodinger equation. It encourages students to participate actively, use their clickers during the competition, and collaborate in recitations to enhance their understanding of the material. The script concludes with a reminder of the next class session and an emphasis on the importance of student involvement.
Mindmap
Keywords
💡Creative Commons license
💡MIT OpenCourseWare
💡Photoelectric effect
💡Photon
💡Work function
💡Quantization
💡Momentum
💡de Broglie wavelength
💡Schrodinger equation
💡Quantum mechanics
💡Binding energy
💡Principal quantum number
Highlights
The photoelectric effect demonstration shows light's particle-like properties and the quantization of energy.
Einstein's explanation of photons having momentum was revolutionary and stemmed from the photoelectric effect.
De Broglie's hypothesis that matter, including electrons, exhibits wave-like properties was a major contribution to quantum mechanics.
The wavelength of particles like electrons is significant when it is on the scale of the object being considered, such as in atoms.
Diffraction patterns observed from electrons confirmed their wave-like behavior, a discovery with significant implications for understanding matter.
The Schrodinger equation is fundamental to quantum mechanics, offering a way to describe the behavior of particles with wave-like properties.
Solving the Schrodinger equation provides insights into the binding energy of electrons and their orbitals, which is crucial for understanding atomic stability.
The development of quantum dots and their applications in fields like medicine and material science underline the practical relevance of quantum mechanics.
Quantum dots exhibit discrete energy levels and can emit light of varying colors depending on their size, with potential uses in medical imaging and diagnostics.
Modern research continues to explore the Schrodinger equation's applications, including in improving computational methods for analyzing large systems like proteins.
The Schrodinger equation's derivation is attributed to Schrodinger's creative insight rather than a direct extrapolation from existing knowledge.
The equation's success in predicting experimental results confirms its validity and its importance in the field of chemistry.
Rydberg's constant simplifies the binding energy calculations from the Schrodinger equation, making it more accessible for students and researchers.
The principal quantum number, a key concept in quantum mechanics, arises naturally from the solutions to the Schrodinger equation.
Upcoming demonstrations of the hydrogen atom spectrum aim to show the practical validation of the Schrodinger equation's predictions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: