SPDF orbitals Explained - 4 Quantum Numbers, Electron Configuration, & Orbital Diagrams
TLDRThis video discusses the sublevels of atomic orbitals, explaining the shapes and electron capacities of s, p, d, and f orbitals. It covers quantum numbers n, l, ml, and ms, and how they determine electron configurations. Examples include identifying quantum numbers for specific electrons and writing electron configurations for elements like phosphorus, illustrating the process with orbital diagrams.
Takeaways
- 🌐 The shape of atomic orbitals is crucial for understanding electron behavior: S orbitals are spherical, P orbitals resemble dumbbells, D orbitals are like clover leaves, and F orbitals have complex shapes.
- 🔢 The number of sublevels in an energy level (n) is equal to the principal quantum number (n). For example, n=1 has 1 sublevel (1s), n=2 has 2 sublevels (2s, 2p), and so on.
- 🚀 The S sublevel can hold up to two electrons, and each orbital can hold up to two electrons. This is a fundamental principle in understanding electron capacity in orbitals.
- 📚 The P block in the periodic table corresponds to groups 13 to 18 and can hold up to six electrons, with three orbitals.
- 🔨 The D block, starting with elements like zinc and copper, can hold up to 10 electrons with five orbitals, as seen in the 3d sublevel.
- 🌟 The F block can hold up to 14 electrons with seven orbitals, illustrating the complexity of higher energy levels.
- 📈 Quantum numbers (n, l, ml, ms) are essential for identifying electron states. n represents the principal energy level, l the sublevel (s, p, d, f), ml specifies the orbital, and ms represents electron spin.
- 🔍 Electron configurations can be determined by filling orbitals according to the Aufbau principle, which states that electrons fill orbitals of lowest energy first.
- 📉 Hund's rule dictates that electrons fill degenerate orbitals one at a time with parallel spins before pairing up, ensuring maximum total spin.
- 🧩 Pauli's exclusion principle states that no two electrons can have the same set of four quantum numbers, ensuring each electron has a unique identity in an atom.
- 📚 Writing electron configurations and orbital notations involves starting with the lowest energy level and filling orbitals in order, reflecting the actual electron distribution in an atom.
Q & A
What is the shape of the s sublevel?
-The s sublevel has a spherical shape.
How many orbitals does the p sublevel have?
-The p sublevel has three orbitals.
What is the maximum number of electrons that the d sublevel can hold?
-The d sublevel can hold up to 10 electrons.
What is the relationship between the principal energy level (n) and the number of sublevels?
-The number of sublevels is equal to the principal energy level (n). For example, when n is 3, there are 3 sublevels: 3s, 3p, and 3d.
How are the orbitals of the s, p, d, and f sublevels designated in terms of quantum numbers?
-For the s sublevel, L = 0; for the p sublevel, L = 1; for the d sublevel, L = 2; and for the f sublevel, L = 3.
What is the value of ml for a p sublevel, and what does it represent?
-The value of ml for a p sublevel varies between -1, 0, and 1. It represents the orientation of the orbital within the sublevel.
Explain the Pauli exclusion principle in the context of quantum numbers.
-The Pauli exclusion principle states that no two electrons can have the same set of four quantum numbers. Each electron in an atom has a unique combination of n, L, ml, and ms.
What is the electron configuration for phosphorus, and how many s electrons does it have?
-The electron configuration for phosphorus is 1s2 2s2 2p6 3s2 3p3. Phosphorus has 6 s electrons.
How are electrons added to degenerate orbitals according to Hund's rule?
-According to Hund's rule, electrons are added one at a time to degenerate orbitals (orbitals with the same energy) until all are half-filled before pairing up.
Describe the process of identifying the four quantum numbers for a given electron, using the example of the 3p5 electron.
-For the 3p5 electron, n is 3 (the principal energy level), L is 1 (since it's a p sublevel), ml varies between -1, 0, and 1 (the fifth electron is in the ml = 0 orbital), and ms is -1/2 (because the fifth electron is spin-down).
Outlines
🌐 Understanding Atomic Orbitals and Quantum Numbers
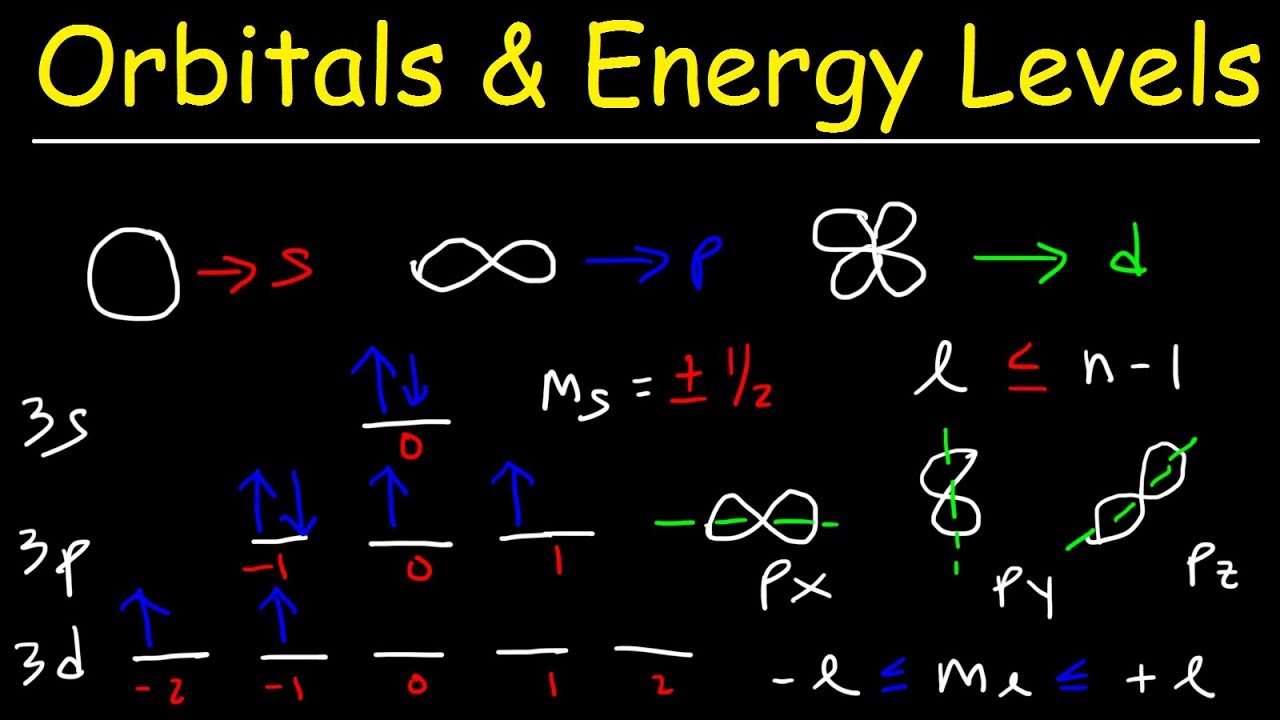
This paragraph introduces the basic concepts of atomic orbitals, specifically focusing on the shapes and characteristics of s, p, d, and f orbitals. It explains that the number of sublevels (s, p, d, f) corresponds to the principal quantum number (n), with s holding up to two electrons, p holding up to six, d holding up to ten, and f holding up to fourteen. The paragraph also delves into the quantum numbers n, l, ml, and ms, which are crucial for identifying the specific state of an electron within an atom. Examples are given to illustrate how to determine these quantum numbers for different electron configurations, such as 3p5 and 4d4. The importance of the Pauli Exclusion Principle is highlighted, which states that no two electrons can have the same set of four quantum numbers.
🔬 Electron Configuration and Orbital Notation
This paragraph continues the discussion on atomic orbitals by focusing on electron configuration and orbital notation. It uses the example of phosphorus, which has 15 electrons, to demonstrate how to write the electron configuration and orbital notation. The explanation covers the filling of orbitals according to the Aufbau Principle and Hund's Rule, emphasizing the importance of filling orbitals in order of increasing energy and ensuring that electrons in degenerate orbitals are filled one at a time. The paragraph concludes with a step-by-step guide on how to fill the orbital diagram for an element, starting with the lowest energy level and moving upwards.
📚 Applying Electron Configuration to Phosphorus
In this paragraph, the focus shifts to applying the principles of electron configuration to the specific case of phosphorus. The video script explains how to determine the electron configuration for phosphorus, which has 15 electrons, by adding up the electrons in each sublevel (1s, 2s, 2p, 3s, 3p) until the total reaches 15. The paragraph also discusses how to answer questions related to the number of electrons in specific orbitals, such as the number of s or p electrons in phosphorus. The video concludes by reviewing the electron configuration for phosphorus and the orbital notation, reinforcing the understanding of how electrons are arranged in the atomic orbitals of an element.
Mindmap
Keywords
💡SP PDF
💡Spherical shape
💡Dumbbell shape
💡Clover leaf
💡Unusual shape
💡Principal energy level
💡Orbital
💡Quantum numbers
💡Pauli's Exclusion Principle
💡Electron configuration
💡Orbital notation
Highlights
The s orbital has a spherical shape, similar to a sphere.
The p orbital has a dumbbell shape and can be drawn in two ways.
The d orbital resembles a clover leaf.
The f orbital has an unusual shape that varies.
The number of energy levels is equal to the number of sublevels.
When n is one, there is only one sublevel (s).
When n is two, there are two sublevels (s and p).
When n is three, there are three sublevels (s, p, and d).
When n is four, there are four sublevels (s, p, d, and f).
The s sublevel can hold up to two electrons.
Each orbital can hold up to two electrons.
The p block in the periodic table corresponds to groups 13 to 18.
The d block starts with elements like zinc, copper, and nickel.
The d sublevel can hold up to 10 electrons and has five orbitals.
The f sublevel can hold up to 14 electrons and has seven orbitals.
The s sublevel corresponds to l=0, p to l=1, d to l=2, and f to l=3.
Four quantum numbers (n, l, ml, ms) are essential for identifying electron configurations.
The Pauli Exclusion Principle states that no two electrons can have the same set of four quantum numbers.
Electron configuration for phosphorus is 1s2 2s2 2p6 3s2 3p3.
Orbital notation and electron filling follow the Aufbau principle and Hund's rule.
Transcripts
in this video we're going to talk about
um the SP PDF
sublevels um what you need to know is
that s has a spherical shape it's like a
sphere P has a dumbbell shape it can be
drawn both ways D is like a clover leaf
and F has some unusual shape which
varies and I really don't want to go
over
that but some things you need to know
the number of ngery levels is equal to
the number of su levels so when n is one
you only have one subl s when n is two
you have two sublevels s n p when n is
three you have three Su levels 3 S 3 p
3D when n is four there are four
sublevels 4 S 4 p 4 d and 4f the S
subl can hold up to two
electrons and you need to know that
every or orbital um can hold up to two
electrons so s has uh one
orbital now in a periodic
table the S block is really the first
two columns group one and group two so
that's the S
block P can hold up to six electrons if
you notice the P Block in the periodic
table it's like Group 13 to group 18 you
can see those six elements there P can
hold up to six electrons and because
every orbital can hold up to two two
electrons uh P has three
orbitals D can hold up to 10
electrons the elements in the D Block
starting with like
um you have like zinc copper nickel
those are in the 3D suev and if you look
at the periodic table there's 10
elements there D can hold up to 10
electrons and so the D suble has five
orbitals F can hold up to 14 electrons
and F has seven orbitals 1 2 3 4 5 6
7 so those are some things you want to
keep in mind by the way whenever you
have the S Sub L is equal to zero for
the P sub L is equal to one for d l is
equal to 2 and for f l is equal to
3 so you need to be familiar with these
four quantum numbers n l ML and Ms we
talked about n already this is the main
principal energy level L represents the
Su which is associated with s p d and
f ml repres it specifies the
orbital s has one orbital and it has a
value of zero P has three orbitals and
it has a value of 1 0 and 1 D has five
orbitals which varies between -2 and two
we're going to talk about that soon Ms
represents the electron spin inside an
orbital you can have an up Arrow which
stands for Plus one2 or you can have a
down arrow which has an electron spin of
negative a
half so let's talk about how to identify
these quantum numbers let's say if you
want to identify the four quantum
numbers for the 3p5
electron and it's going to be this
number n is
three now P will tell you what the value
of L is keep in mind for S L is zero for
p l is one for d l is two for f l is
three now P has uh three orbitals as we
talked about and because L is one ml is
going to vary between NE 1 Z and
one now we want to we're focused on the
fifth electron so here's the first
electron second third fourth fifth the
fifth electron lands in this orbital
where ml is zero so therefore ml is Zer
Ms is negative a half because the fifth
Arrow points down you always start by
drawing the arrows up and then down so
those are the four quantum numbers that
corresponds to the 3p5
electron let's try two more examples
let's try 4
D4 n is
4 and for d l is two because for S L is
zero for p l is one and for f l is three
now the d sub Lev has five
orbitals and so ml can vary between -2 1
0 1 and two because L is
two so here's the first Arrow second
third fourth we're interested in in the
fourth arrow and it landed on the
orbital that has a value of one and
because it's an up Arrow the spin is
positive2 all right for the sake of
practice let's try one more example
let's focus on the 5f 13 electron so n
is
five L is zero for S L is one for p l is
2 for d for f l is
three and for the F suble there are
seven
orbitals
and ml can vary between -3 and 3 because
l
is3 so we're interested in the 13th
electron 1 2 3 4 5 6 7 8 9 10 11 12 13
there it is and it landed in this
orbital so MLS
2 and it's a down arrow so the electron
spin is negative half so that's how you
could find the four quantum numbers uh
given the
electron now Paul's exclusion principle
states that no two electrons can have
the same set of four quantum numbers as
you can see these quantum numbers are
unique for each electron this one
electron has its a unique set of four
quantum numbers so if you're given these
four quantum numbers you can identify
What electron we're talking about let's
try that so let's say for example
example if uh n is 3 l is 2 ml is 1 and
Ms is negative a half What electron are
we talking about what which electron is
identified by these four um unique
quantum
numbers so we know we're in the third
enery
level when L is zero It's s when L is
one it's p when L is 2 it's D so we're
in the 3D suev D has five
orbitals and because L is 2 it varies
between -2 and 2 excuse
me now we know that the electron is in
this orbital because ml is one and we
know the arrow has to be a down arrow so
let's count it 1 2 3 start with the up
arrows four five 6 7 8 9 there's our
down arrow so these four quantum numbers
Cor Corr responds to the 3d9
electron now let's talk about electron
configuration and orbital notations and
so forth let's say if you want to write
the electron configuration
for let's go with uh
phosphorus now if I remember correctly I
believe phosphorus has 15
electrons let me just take a minute and
verify that with periodic table and yeah
that's
correct now the first energy level only
has one suel the second NG level has two
sublevels the third NG level has three
Su levels so 3s3 p3d the fourth NG level
has four Su
levels so let's say if you want to write
the electron configuration for
phosphorus the configuration the
exponents has to add up to this atomic
number now keep in mind s can hold up to
two electrons P can hold up to six D can
have up to 10 F can have up to 14 and
we're going to write it until the
exponents add up to
15 so let's begin so One S can hold up
to two electrons 2s can also hold up to
two 2p can hold up to six
electrons and 3 S can hold up to two so
right now we have 2 + 2 which is 4 + 6
which is 10 10 + 2 12 we only need three
more 3p can hold up to six but because
we only need three more we're going to
stop at 3p3 this is the electron
configuration for
phosphorus now let's say if you're given
a question and they ask you how many s
electrons are in phosphorus after you
write the configuration you can answer
the question so there are six s
electrons in phosphorus because if you
add up the exponents you get six if they
ask you hey how many P electrons are on
phosphorus simply add the P electrons 6
+ 3 there are n p electrons in
phosphorus now let's refresh the page
but let's keep the information that we
have so we said the electron
configuration for phosphorus is 1 S2 2
S2 2 P6 3 S2 and 3 P3 but now let's
write the orbital notation um for
phosphorus or the orbital
diagram so this is the 1s orbital s has
only one orbital here we have 2 s 3s
notice I keep it in the same column then
to the right of that just above 2s we
have 2p which has three orbitals and
3p now as you go up the potential energy
increases now according to offb
principle um you need to add the
electrons in increase in order which
means you start from the lowest energy
level and then you go up to the the
highest energy level so we have to start
with 1s we put the first electron here
we don't put the next one in 2s you have
to put the next one in ons you have to
go in order that's off boss principle
2s2 is filled now according to Hun's
rule whenever you're filling electrons
in degenerate orbitals you have to fill
them one at a time the word degenerate
means that the energy is equal so these
three orbitals have equal energy because
they're at the same height so therefore
they are degenerate
orbitals because they have the same
energy so whenever you're adding
electrons here you add it one at a time
according to hun rule so 1 2 3 4 five
six 2p6 is filled so next according to
off boss principle we move into 3s not
3p because we have to go in order of
increase in energy or increase in
potential energy so 3s2 and then based
on Hun's rule for degenerate orbitals
which are energy levels at the which are
orbitals that have the same energy we
have to fill these um orbitals one at a
time so 1 2 3 that's how you fill the
orbital diagram for an element write the
electron configuration
first then put the arrows in so that's
it for this video um I think we covered
a lot and uh I have other videos on
quantum numbers so feel free to search
YouTube for those and uh so that's all I
got for today that's my two cents and
Browse More Related Video

Quantum Numbers, Atomic Orbitals, and Electron Configurations

Quantum Numbers
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers

Electron Configuration

7.3 Electron Configuration | High School Chemistry

How to Write the Electron Configuration of an Element | Study Chemistry With Us
5.0 / 5 (0 votes)
Thanks for rating: