Quantum Numbers, Atomic Orbitals, and Electron Configurations
TLDRThe video script introduces quantum numbers as key to understanding electron arrangement in atoms. It explains the four quantum numbers: principal (n), angular momentum (l), magnetic (m_l), and spin (m_s), which together define electron orbitals and their energies. The script details the shapes of s, p, d, and f orbitals, and how electrons fill these orbitals according to the Aufbau principle and Hund's rule. The Pauli exclusion principle is highlighted, emphasizing that no two electrons in an atom can have the same set of quantum numbers. The video concludes with tips on determining electron configurations and the properties of paramagnetic and diamagnetic atoms.
Takeaways
- 🌟 Electrons exhibit both particle and wave-like properties, which influence their arrangement in atoms.
- 📍 Quantum numbers are used to describe the location and energy of electrons in atomic orbitals, which are regions of probability where electrons can be found.
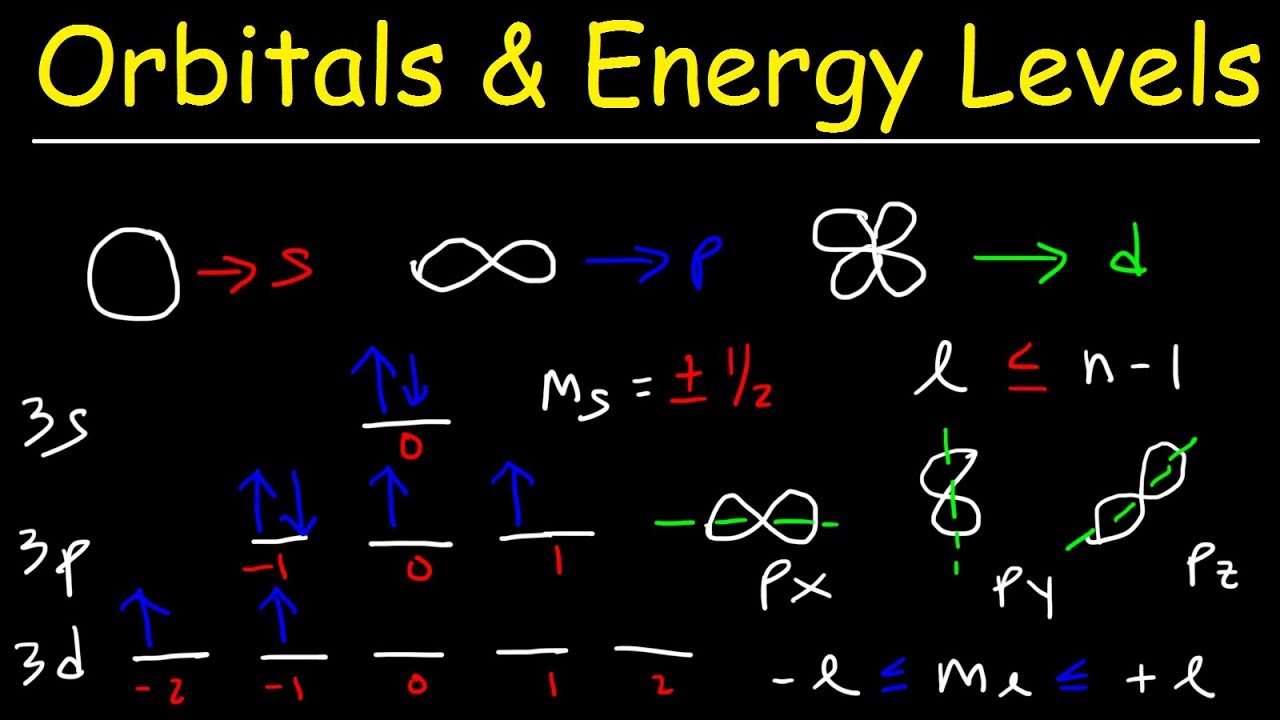
- 🔢 There are four quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (m_l), and the spin quantum number (m_s).
- 💫 Orbitals have different shapes: s is spherical, p has three lobes, d is more complex with up to five shapes, and f is highly intricate with seven shapes per energy level.
- 📈 The principal quantum number (n) determines the energy level of an electron, with higher n values indicating greater distance from the nucleus.
- 🔄 The angular momentum quantum number (l) ranges from 0 to n-1 and describes the shape of the orbital.
- 🌐 The magnetic quantum number (m_l) can vary from -l to +l, indicating specific orbitals within an energy level.
- 🔄 The spin quantum number (m_s) can be either +1/2 or -1/2, representing the two possible spin states of an electron.
- 🚫 The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers, meaning each electron has a unique set.
- 📊 The Aufbau principle outlines the order in which atomic orbitals are filled, starting with the lowest energy orbitals and moving to higher energy levels.
- 📌 The electron configuration of an element can be determined by following the Aufbau principle and considering the number of electrons each orbital can hold.
Q & A
What are quantum numbers and why are they important in understanding atomic structure?
-Quantum numbers are a set of four numerical values that describe the distinct atomic orbitals in which electrons can be found within an atom. They are crucial for determining the location, energy, and behavior of electrons, and thus help in understanding and predicting the electronic structure of atoms.
What are the four quantum numbers and what do they represent?
-The four quantum numbers are: (1) Principal quantum number (n), which represents the energy level of the electron; (2) Angular momentum quantum number (l), which describes the shape of the orbital; (3) Magnetic quantum number (m_l), which determines the specific orbital within a given energy level; and (4) Spin quantum number (m_s), which indicates the electron's spin state.
How do s, p, d, and f orbitals differ in shape and the number of electrons they can hold?
-S orbitals are spherical and can hold a maximum of two electrons. P orbitals have a lobed shape extending on three axes and can also hold up to two electrons. D orbitals have a more complex shape with five distinct regions and can accommodate up to ten electrons. F orbitals are even more complex with seven regions and can hold up to fourteen electrons.
What is the significance of the Pauli exclusion principle in relation to quantum numbers?
-The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that an orbital can hold a maximum of two electrons with opposite spins, ensuring that each electron in an atom has a unique set of quantum numbers.
What is the Aufbau principle and how does it help in determining electron configurations?
-The Aufbau principle dictates the order in which atomic orbitals are filled with electrons based on their relative energies. Orbitals further from the nucleus have higher potential energy and are filled first. This principle helps in predicting the electron configuration of any atom by filling orbitals from the lowest energy level upwards.
How does Hund's rule relate to the filling of orbitals?
-Hund's rule states that electrons will fill each orbital of the same energy level singly and with parallel spins before any orbital is doubly occupied. This rule helps in determining the arrangement of electrons in degenerate orbitals (orbitals with the same energy).
What is the electron configuration of a neutral chlorine atom?
-The electron configuration of a neutral chlorine atom, which has 17 electrons, is 1s² 2s² 2p⁶ 3s² 3p⁵. This configuration shows the distribution of electrons in the various orbitals according to the Aufbau principle.
How can you quickly determine the electron configuration of an element using the periodic table?
-You can quickly determine the electron configuration of an element by moving from left to right and top to bottom on the periodic table, starting from the noble gas core of the previous period. Each element adds one more electron, which fills the orbitals in the order of increasing energy levels.
What are paramagnetic and diamagnetic atoms, and how do their electron configurations relate to magnetic fields?
-Paramagnetic atoms have unpaired electrons in their orbitals and are attracted by a magnetic field due to the presence of magnetic moments. Diamagnetic atoms, on the other hand, have all their electrons paired, resulting in no net magnetic moment and thus are not affected by magnetic fields.
How can you visually depict the filling of orbitals using orbital diagrams?
-Orbital diagrams visually represent the filling of electrons in orbitals according to their energy levels and shapes. They follow the Aufbau principle and Hund's rule, showing the distribution of electrons and their spins within the orbitals.
What is the significance of the shapes of atomic orbitals in determining the behavior of electrons?
-The shapes of atomic orbitals dictate the regions in space where electrons are most likely to be found. These probabilities influence chemical bonding, as the orientation and overlap of orbitals play crucial roles in the formation of chemical bonds between atoms.
Outlines
🌟 Understanding Quantum Numbers and Atomic Orbitals
This paragraph introduces the concept of quantum numbers and their role in determining the arrangement of electrons in an atom. It explains the four quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (m_l), and the spin quantum number (m_s). The paragraph describes how these numbers define the energy level, shape, orientation, and spin of atomic orbitals. It also discusses the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers. The electron configuration process, including the Aufbau principle and Hund's rule, is outlined, providing examples with hydrogen, helium, and chlorine atoms.
📊 Visualizing Electron Configurations and Orbital Filling
This paragraph delves into the practical application of electron configurations and the filling of orbitals. It explains how to determine the electron configuration of an atom by following the Aufbau principle and using the periodic table as a guide. The paragraph introduces the concept of s, p, d, and f blocks in the periodic table and how they correspond to different types of orbitals. It also provides a method for quickly determining electron configurations by using noble gas cores and listing the valence electrons. The paragraph concludes with a discussion on paramagnetic and diamagnetic properties of atoms, relating them to the presence of unpaired electrons in orbital diagrams.
Mindmap
Keywords
💡Quantum Numbers
💡Electrons
💡Atomic Orbitals
💡Principal Quantum Number (n)
💡Angular Momentum Quantum Number (l)
💡Magnetic Quantum Number (m_sub_l)
💡Spin Quantum Number (m_sub_s)
💡Pauli Exclusion Principle
💡Aufbau Principle
💡Electron Configuration
💡Hund's Rule
💡Paramagnetic and Diamagnetic Atoms
Highlights
Electrons exhibit both particle and wave characteristics, which is crucial for understanding their arrangement in atoms.
The arrangement of electrons in an atom is described by four quantum numbers that define different atomic orbitals.
Atomic orbitals are regions of probability where electrons can be found, and they come in s, d, p, and f varieties with distinct shapes.
Each orbital can hold up to two electrons, and the more electrons an atom has, the more orbitals are needed to accommodate them.
The principal quantum number (n) represents the energy level of an electron and increases with distance from the nucleus.
The angular momentum quantum number (l) determines the shape of the orbital, with values ranging from 0 to n-1.
The magnetic quantum number (m_l) specifies a particular orbital within an energy level and can have values from -L to L.
The spin quantum number (m_s) is either +1/2 or -1/2, indicating the spin direction of an electron.
No two electrons in an atom can have the same set of four quantum numbers due to the Pauli exclusion principle.
The Aufbau principle dictates the order in which electrons fill orbitals, starting with the lowest energy levels.
The electron configuration of an atom can be determined by filling orbitals according to their energy levels.
The 1s orbital is the lowest energy orbital and is filled first in the periodic table.
Hund's rule states that electrons should be distributed singly across orbitals of the same energy level before pairing up.
Atoms with unpaired electrons are paramagnetic and attracted by a magnetic field, while those with all paired electrons are diamagnetic and not affected by magnetic fields.
Orbital diagrams visually depict how electrons are distributed among the orbitals, following Hund's rule for filling.
The s, p, d, and f blocks of the periodic table correspond to the types of orbitals that elements' valence electrons occupy.
The electron configuration of an element can be quickly determined by moving left to right and up to down on the periodic table.
Transcripts
Browse More Related Video

7.4 Quantum Numbers | High School Chemistry
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers

Learning Outcomes (f), (g), (h) of Atomic Structure [JC H2 Chemistry]

SPDF orbitals Explained - 4 Quantum Numbers, Electron Configuration, & Orbital Diagrams

Lecture 13 - Quantum numbers

Quantum Numbers
5.0 / 5 (0 votes)
Thanks for rating: