Aleks Writing net ionic equations
TLDRThis educational video script explains the concept of net ionic equations in chemistry. It uses the analogy of net pay to describe how only certain ions in a reaction contribute to the final product, while others are 'leftovers' and do not participate. The script provides a step-by-step guide on writing net ionic equations, focusing on the dissolution of ionic compounds into ions, identifying the product, and recognizing which ions are spectator ions that do not affect the reaction. The process is illustrated with an example involving magnesium, hydroxide, and nitrate ions, culminating in the formation of magnesium hydroxide as a solid product.
Takeaways
- 📚 Net ionic equations represent the simplified chemical reactions where only the species that actually participate in the reaction are shown.
- 💧 The term 'aqueous' refers to substances that are dissolved in water and dissociate into ions.
- 🛁 When an ionic compound dissolves in water, it breaks apart into its constituent positive and negative ions.
- 🚫 Not all substances in the water contribute to the final product; some are 'leftovers' and are not included in the net ionic equation.
- 🔬 The process involves identifying which ions are present in the solution and which ones form a product that does not dissolve in water.
- 🧪 In the example given, magnesium and hydroxide ions combine to form magnesium hydroxide, a solid that precipitates out of the solution.
- 🔄 The net ionic equation omits the ions that do not participate in the reaction, known as spectator ions.
- ⚖️ Balance is important in writing net ionic equations, ensuring that the number of each type of ion is the same on both sides of the reaction.
- 📝 The script demonstrates the process of writing a net ionic equation step by step, starting with identifying the ions in solution and ending with the formation of the solid product.
- 📉 The example uses magnesium nitrate and sodium hydroxide reacting in water to form magnesium hydroxide solid and sodium nitrate in solution.
- 🔑 Understanding net ionic equations is key to grasping the essence of chemical reactions in solutions, focusing on the changes that occur rather than the initial concentrations of reactants.
Q & A
What is a net ionic equation?
-A net ionic equation is a chemical equation that shows only the species that actually participate in the reaction and form the product, excluding the spectator ions that do not contribute to the reaction.
What does 'net pay' have to do with net ionic equations?
-The term 'net pay' is used as an analogy to explain net ionic equations. Just as net pay is what remains after taxes, a net ionic equation shows what remains (reacts) after excluding the spectator ions.
What does 'aqueous' mean in the context of chemistry?
-'Aqueous' refers to a substance that is dissolved in water. In the context of writing net ionic equations, it indicates that the compound is in the form of ions in water.
How does an ionic compound behave when dissolved in water?
-When an ionic compound is dissolved in water, it dissociates into its constituent positive and negative ions.
What is the role of magnesium nitrate in the example given in the script?
-In the example, magnesium nitrate is an ionic compound that, when dissolved in water, dissociates into magnesium ions (Mg^2+) and nitrate ions (NO3^-).
What is a polyatomic ion and how does it behave in water?
-A polyatomic ion is a group of atoms that are bonded together and act as a single ion. For example, nitrate (NO3^-) is a polyatomic ion that stays intact and does not break apart when dissolved in water.
What is the significance of the magnesium and hydroxide ions in the reaction?
-Magnesium and hydroxide ions are significant because they react to form magnesium hydroxide, a solid that is insoluble in water and thus precipitates out of the solution.
What are spectator ions in a chemical reaction?
-Spectator ions are ions that are present in the reaction but do not participate in the formation of the product. They remain unchanged before and after the reaction.
How do you identify spectator ions in a net ionic equation?
-Spectator ions are identified by their presence on both sides of the reaction with the same charge and composition, indicating they do not participate in the reaction.
What is the final product of the reaction described in the script?
-The final product of the reaction described in the script is magnesium hydroxide (Mg(OH)2), which is a solid that precipitates out of the solution.
Why is the net ionic equation important in understanding chemical reactions?
-The net ionic equation is important because it simplifies the chemical reaction by showing only the species that actually react to form the product, making it easier to understand the essence of the reaction.
Outlines
🧪 Understanding Net Ionic Equations
This paragraph introduces the concept of net ionic equations in the context of chemistry. It explains that a net ionic equation represents the substances that are actually involved in a chemical reaction, excluding those that are present but do not contribute to the formation of the product. The paragraph uses the analogy of net pay to illustrate the idea of what remains after all deductions. It then gives an example of how ionic compounds dissolve in water to form ions, which are the active participants in chemical reactions. The focus is on identifying which ions will combine to form a solid product, which is not soluble in water, and thus will not dissociate back into ions.
🔍 Identifying Spectator Ions in Reactions
The second paragraph delves into the role of spectator ions in chemical reactions. These are ions that appear on both sides of the reaction and do not participate in the formation of the product. The paragraph provides a step-by-step explanation of how to identify spectator ions by comparing the ions present before and after the reaction. It uses the example of a reaction involving magnesium, hydroxide, sodium, and nitrate ions, where magnesium and hydroxide ions combine to form a solid product, magnesium hydroxide, which is insoluble in water. The remaining ions, sodium and nitrate, are identified as spectator ions because they do not participate in the reaction. The paragraph concludes with the writing of the net ionic equation, which includes only the ions that are involved in the formation of the product and excludes the spectator ions.
Mindmap
Keywords
💡Net Ionic Equations
💡Aqueous
💡Ionic Compounds
💡Spectator Ions
💡Dissociation
💡Product
💡Coefficient
💡Solid
💡Soluble
💡Mg^2+ and OH^-
💡Net Reaction
Highlights
Net ionic equations represent what is left over after everything cancels out, similar to net pay.
Aqueous refers to substances dissolved in water, breaking down into ions.
Ionic compounds dissociate into positive and negative ions when dissolved in water.
Not all substances in water contribute to the product in a reaction.
The product of a reaction is what is actually made, not just what is present in the solution.
Magnesium nitrate dissociates into magnesium ions and nitrate ions in water.
Nitrate ions remain intact as a polyatomic ion in water.
Sodium ions and hydroxide ions are also present in the solution and can dissociate.
Magnesium and hydroxide ions can combine to form a solid product, magnesium hydroxide.
Sodium and nitrate ions do not participate in the formation of the product and are considered spectator ions.
Sodium and nitrate ions are present on both sides of the reaction and are not involved in the net reaction.
The net ionic equation includes only the ions that participate in the formation of the product.
Magnesium hydroxide is the solid product and does not dissociate in water.
The net ionic equation is written by identifying the ions that do not cancel out and form the product.
The net ionic equation for the reaction is Mg^2+(aq) + 2OH^-(aq) yields Mg(OH)2(s).
Understanding net ionic equations helps in identifying the actual products of a chemical reaction.
The process of writing net ionic equations involves recognizing which ions are spectator ions and which contribute to the product.
Transcripts
Browse More Related Video
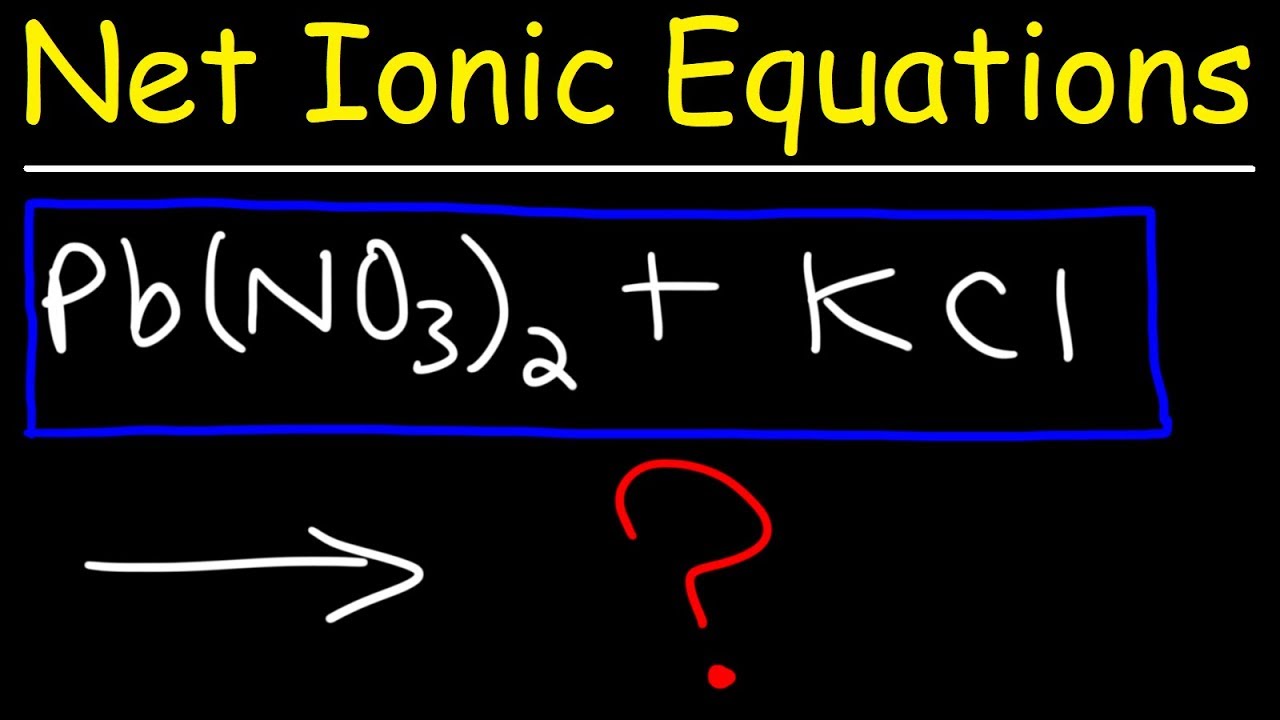
How To Write Net Ionic Equations In Chemistry - A Simple Method!

Molecular, complete ionic, and net ionic equations | AP Chemistry | Khan Academy

How to Write Complete Ionic Equations and Net Ionic Equations

Introduction to Double Replacement Reactions

Lesson 13 - Ions And Ionic Charge (Chemistry Tutor)

Net Ionic Equation Worksheet and Answers
5.0 / 5 (0 votes)
Thanks for rating: