How to Write Complete Ionic Equations and Net Ionic Equations
TLDRThe video script outlines a step-by-step guide to predicting chemical products, balancing chemical equations, and understanding solubility. It begins with the concept of 'inner with inner, outer with outer' to predict reactants and products, using lead and chlorine to form lead chloride and potassium with nitrate to form potassium nitrate. The script then demonstrates how to balance the chemical equation by listing elements and ensuring polyatomic ions like nitrate remain intact. Following this, solubility is determined using a solubility table, identifying KCl and nitrate as aqueous and lead chloride as a solid. The complete ionic equation is written by breaking down aqueous compounds into ions, leaving solids intact. Finally, the net ionic equation is derived by canceling out spectator ions present on both sides of the reaction. The script encourages viewers to persevere through challenging concepts and offers additional resources for further understanding.
Takeaways
- 🧠 **Reactant Combination**: Inner elements (like lead and chlorine) combine to form one product, and outer elements (like potassium and nitrate) combine to form another product.
- 🔋 **Charge Placement**: When writing compounds, place the metal (positive charge) first, followed by the nonmetal (negative charge).
- 🔢 **Balancing Equations**: Balance chemical equations by ensuring elements on opposite sides are equivalent, keeping polyatomic ions intact for easier balancing.
- 📊 **Solubility Identification**: Use a solubility table to determine if compounds are soluble or insoluble, noting exceptions for certain elements like lead.
- 🌊 **Aqueous Compounds**: Compounds identified as soluble are considered aqueous and will dissociate into ions in solution.
- 📚 **Solubility Rules**: Know the solubility rules for different types of ions and compounds, such as halogens being soluble except for a few metals.
- 🧩 **Ionic Equations**: Write complete ionic equations by breaking apart aqueous compounds into their respective ions, leaving solids and liquids intact.
- ⚖️ **Spectator Ions**: In net ionic equations, cancel out ions that appear on both sides of the reaction, as they do not participate in the reaction (e.g., potassium and nitrate ions).
- ➗ **Net Ionic Equation**: The final net ionic equation includes only the ions necessary to form the solid product, excluding spectator ions.
- 🔬 **Practical Application**: Understanding solubility and how to write ionic equations is crucial for predicting reaction outcomes and is a fundamental concept in chemistry.
- 💪 **Perseverance**: The speaker encourages viewers to keep pushing forward even when concepts seem challenging, emphasizing that persistence leads to success.
Q & A
What is the concept of 'inner with inner, outer with outer' in predicting chemical products?
-The concept refers to the combination of elements within the same group (inner elements) or different groups (outer elements) to form products. Inner elements combine to form one product, and outer elements combine to form another product.
How should you write the charges of metals and nonmetals in a chemical formula?
-You should write the metal (positive charge) first, followed by the nonmetal (negative charge).
What is the role of the subscript in a chemical formula?
-The subscript indicates the number of atoms of an element in a molecule. It is used to balance the charges between elements in a compound.
How do you balance a chemical equation?
-You balance a chemical equation by ensuring that the number of each type of atom on the reactant side equals the number on the product side. You may need to place coefficients in front of molecules to achieve this balance.
Why is it important to keep polyatomic ions intact when balancing chemical equations?
-Polyatomic ions should be kept intact to simplify the balancing process, especially when they appear the same on both sides of the equation.
What does it mean for a compound to be 'soluble'?
-A compound is considered soluble if it dissolves in water to form an aqueous solution. Solubility is often determined by consulting a solubility table.
How do you identify the solubility of a compound using a solubility table?
-You look up the elements or polyatomic ions of the compound in the solubility table and check if there are any exceptions to the general solubility rules. If not, the compound is considered soluble (aqueous).
Why are solids and liquids not broken apart in an ionic equation?
-Solids and liquids are not broken apart in an ionic equation because they do not dissociate into ions when dissolved in water. Only aqueous compounds are broken down into their constituent ions.
What is a complete ionic equation?
-A complete ionic equation, also known as a total ionic equation, is an equation that shows all the aqueous reactants and products in ionic form, with solids and liquids left intact.
What are spectator ions and why are they canceled out in the net ionic equation?
-Spectator ions are ions that appear on both the reactant and product sides of a reaction and do not participate in the reaction. They are canceled out in the net ionic equation to simplify it and show only the ions that are directly involved in the reaction.
What is the final step in writing a net ionic equation?
-The final step is to cancel out the spectator ions from the complete ionic equation, leaving only the ions that are directly involved in the reaction to form the products.
Why is it important to understand solubility and how to read a solubility table?
-Understanding solubility and how to read a solubility table is crucial for predicting the products of a reaction, writing correct ionic equations, and determining the feasibility of a chemical reaction in an aqueous environment.
What encouragement does the speaker offer to those who find these concepts challenging?
-The speaker encourages learners to persevere, stating that feeling like giving up often means you are closer to success. They share their own experiences and encourage continuous effort and progress.
Outlines
🧪 Predicting Products and Balancing Equations
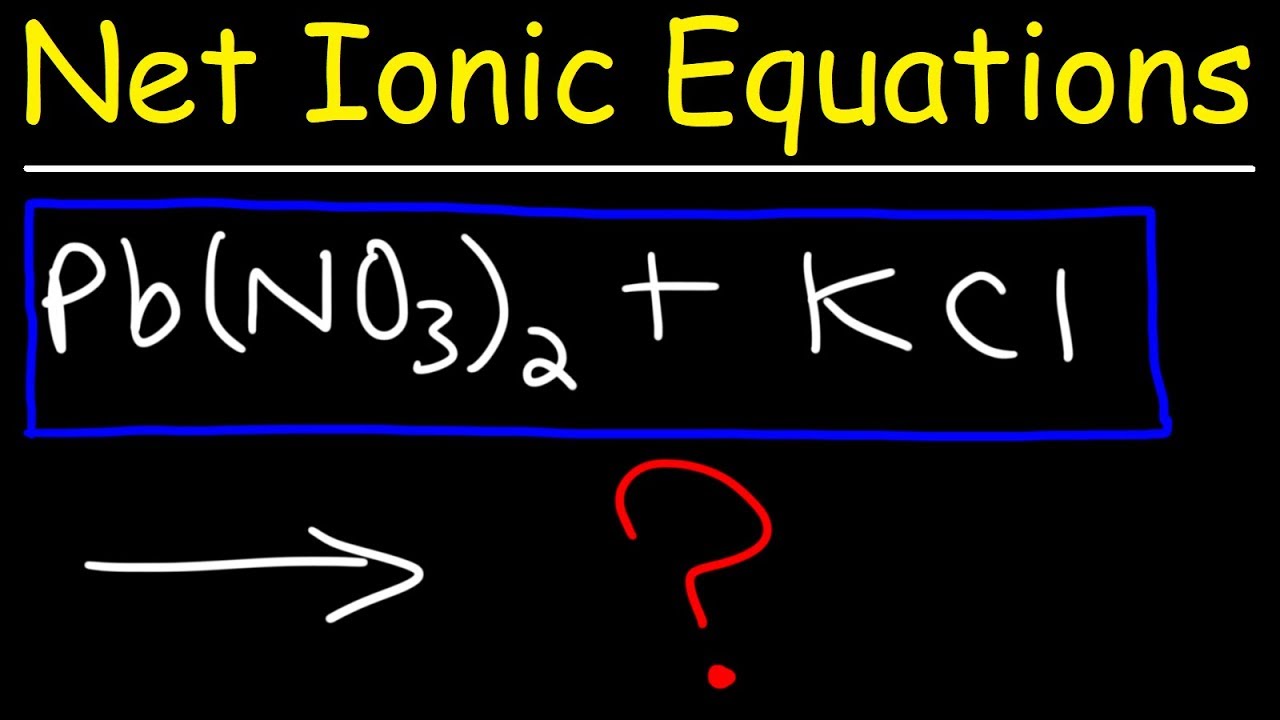
The first paragraph explains the process of predicting the products of a chemical reaction and balancing the chemical equation. It begins with the concept of 'inner with inner and outer with outer', which refers to the combination of elements to form products. The example uses lead and chlorine to form lead chloride and potassium and nitrate to form potassium nitrate. The paragraph then details how to balance the equation by listing elements on opposite sides and ensuring polyatomic ions like nitrate are kept intact. The process involves adjusting the coefficients to balance the elements, resulting in a balanced equation.
📊 Identifying Solubility and Writing Ionic Equations
The second paragraph focuses on identifying the solubility of compounds using a solubility table and writing the complete ionic equation. It explains that solubility is determined by the position of elements on the solubility table, with exceptions noted for certain metals. The paragraph outlines that potassium (being a Group one element) and nitrate are always soluble, while lead chloride is an exception and thus considered a solid. Following solubility identification, the paragraph details breaking apart aqueous compounds into ions, leaving solids and liquids intact. The complete ionic equation is then constructed by combining reactants and products ions. The process concludes with simplifying the equation by canceling out spectator ions, which are present on both sides of the reaction, to obtain the net ionic equation. The paragraph ends with encouragement to persevere through challenging concepts and a reminder to check additional resources for further assistance.
Mindmap
Keywords
💡Reactants
💡Products
💡Balancing Chemical Equations
💡Solubility Table
💡Aqueous
💡Ionic Equation
💡Net Ionic Equation
💡Spectator Ions
💡Charge
💡Polyatomic Ions
💡Metal and Nonmetal
Highlights
Predict the products by combining inner elements (lead and chlorine) to form lead chloride and outer elements (potassium and nitrate) to form potassium nitrate.
Place a positive charge metal first, followed by the negative charge nonmetal when writing compounds.
Lead has a 2+ charge and chlorine has a -1 charge, resulting in the formation of lead chloride.
Potassium has a +1 charge and nitrate has a -1 charge, leading to the formation of potassium nitrate with balanced charges.
When balancing chemical equations, list elements on opposite sides and keep polyatomic ions like nitrate intact.
Balance the equation by ensuring the number of each element is the same on both the reactant and product sides.
Use a solubility table to identify the solubility of each compound in the reaction.
Halogens like chlorine are typically soluble except with the three metals at the bottom of the group.
Potassium, being in Group 1, is always soluble, making potassium chloride aqueous.
Nitrate is soluble with no exceptions, so potassium nitrate is also aqueous.
Lead chloride is a solid since lead is an exception to the solubility rule for halogens.
Write the complete ionic equation by breaking apart aqueous compounds into their separate ions.
Do not break apart solids or liquids in the complete ionic equation.
Combine the ions from the reactants and products to write the complete ionic equation.
Cancel out spectator ions (ions present on both sides of the equation) to obtain the net ionic equation.
The net ionic equation shows the ions needed to form the final solid product.
Potassium and nitrate are spectator ions in this reaction and cancel each other out.
Chlorine and lead ions are required to form the final solid product, lead chloride.
For more help with solubility and understanding solubility tables, check out the latest video and download the solubility table.
Keep moving forward and don't give up, as persistence is key to success in understanding these concepts.
Transcripts
Browse More Related Video
How To Write Net Ionic Equations In Chemistry - A Simple Method!

Molecular, complete ionic, and net ionic equations | AP Chemistry | Khan Academy

Introduction to Double Replacement Reactions

Chemical Reactions (1 of 11) Double Replacement Reactions, An Explanation

How to Predict Products of Chemical Reactions | How to Pass Chemistry

Net Ionic Equation Worksheet and Answers
5.0 / 5 (0 votes)
Thanks for rating: