Net Ionic Equation Worksheet and Answers
TLDRThis educational video script delves into the intricacies of writing net ionic equations for various types of chemical reactions, including double replacement, precipitation, acid-base, gas evolution, and single replacement reactions. The transcript begins by illustrating a double replacement reaction between magnesium chloride and silver nitrate, highlighting the formation of magnesium nitrate and silver chloride, and emphasizing the importance of understanding solubility rules to predict reaction outcomes. The video then progresses to discuss acid-base reactions, gas evolution reactions, and single replacement reactions, using examples to demonstrate how to balance equations and identify spectator ions. The activity series is introduced as a tool to predict the feasibility of single replacement reactions. Throughout the script, the focus is on practical application, with clear instructions on how to break down complex reactions into simpler, more manageable steps, ultimately providing viewers with a solid foundation in chemical reaction mechanisms.
Takeaways
- 🌟 **Double Replacement Reactions**: When two compounds exchange ions, forming two new compounds, it's a double replacement reaction.
- 💧 **Precipitation Reactions**: Occur when the reaction produces an insoluble solid (precipitate) that does not dissolve in water.
- 🌬️ **Gas Evolution Reactions**: Involve the production of a gas, such as carbon dioxide, which escapes from the solution.
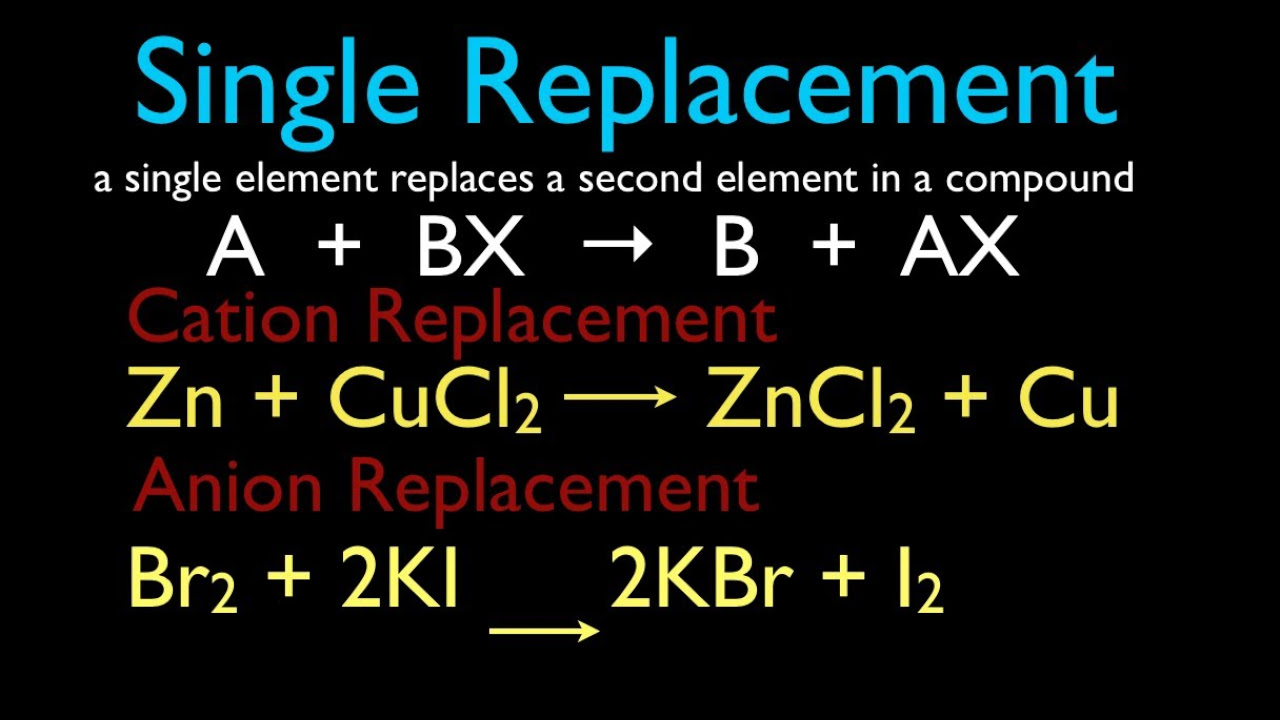
- 🔄 **Single Replacement Reactions**: Involve a more reactive element displacing a less reactive element from its compound.
- ⚖️ **Balancing Equations**: Ensure the number of atoms for each element is the same on both sides of the equation.
- 📋 **Writing Net Ionic Equations**: Separate substances in aqueous phase into ions and eliminate spectator ions that do not participate in the reaction.
- 🚫 **Solubility Rules**: Key to predicting whether a reaction will produce a precipitate or remain soluble in water.
- 🔍 **Activity Series**: A list that ranks metals by their reactivity, used to predict if a single replacement reaction will occur.
- 🔵 **Group 1 Metals**: Always soluble in water, including sodium, lithium, and potassium.
- 🔴 **Polyatomic Ions**: Groups of atoms that act as a single ion with a charge, like nitrate and sulfate.
- ✅ **Product Formation**: In double replacement reactions, predict products by pairing the cations and anions of the reactants to form new compounds.
Q & A
What type of reaction occurs when an aqueous solution of magnesium chloride reacts with silver nitrate?
-A double replacement reaction occurs because the reaction is in the form A B + C D, where magnesium (A) pairs with nitrate (D) to form magnesium nitrate (Mg(NO3)2) and silver (C) pairs with chloride (B) to form silver chloride (AgCl).
What is the charge of magnesium and how does it pair with the nitrate ion to form magnesium nitrate?
-Magnesium has a +2 charge and pairs with the nitrate ion, which has a -1 charge, to form magnesium nitrate using the crisscross method, resulting in the formula Mg(NO3)2.
Why is silver chloride considered insoluble in water?
-Silver chloride is considered insoluble in water due to the solubility rules which state that chlorides, bromides, and iodides are generally soluble except with silver, lead, and mercury(II) cations.
How do you balance the equation for the reaction between magnesium chloride and silver nitrate?
-The equation is balanced by ensuring that the number of atoms for each element on both sides of the equation is equal. For this reaction, placing a '2' in front of AgNO3 and MgCl2 balances the equation as there are two nitrate ions and two chloride ions involved.
What is the net ionic equation for the reaction between sulfuric acid and aqueous sodium hydroxide?
-The net ionic equation for the reaction between sulfuric acid (H2SO4) and aqueous sodium hydroxide (NaOH) is H+ + OH- → H2O, which represents the formation of water from the reaction of hydrogen ions and hydroxide ions.
How do you determine if a reaction between a weak acid and a strong base will occur?
-You can determine if a reaction will occur by looking at the reactivity of the acid and base. In the case of a weak acid and a strong base, the strong base will typically react with the weak acid to form water and a salt, provided that the weak acid is strong enough to donate a proton (H+) to the base.
What is a gas evolution reaction and how is it represented in the total ionic equation?
-A gas evolution reaction is a type of chemical reaction where a gas is produced. In the total ionic equation, it is represented by showing the formation of the gas from the reaction, such as the production of CO2 gas from the reaction of sodium carbonate with hydrochloric acid.
What are the general solubility rules for sulfates?
-Sulfates are generally soluble except when bonded to certain metal cations like calcium, lead, barium, and strontium, which have +2 charges.
How do you determine if a single replacement reaction will occur?
-A single replacement reaction will occur if the element in the reaction (usually a metal or nonmetal) on the left is more reactive according to the activity series than the element it is replacing in the compound on the right.
What is the general form of a single replacement reaction and how does it differ from a double replacement reaction?
-The general form of a single replacement reaction is A + BC → AC + B, where A displaces B from the compound BC. This differs from a double replacement reaction, which involves the exchange of components between two compounds, typically in the form A B + C D → A D + B C.
What is the activity series and how is it used in chemistry?
-The activity series is a list of metals arranged in order of decreasing reactivity. It is used to predict the outcomes of possible reactions between metals and other substances, such as whether a metal can displace another metal from a compound in a single replacement reaction.
Outlines
🔍 Understanding Double Replacement Reactions
This paragraph introduces the topic of writing net ionic equations for various types of chemical reactions, including double replacement, precipitation, acid-base, gas evolution, and single replacement reactions. It uses the example of an aqueous solution of magnesium chloride reacting with silver nitrate to explain the concept of double replacement reactions. The process involves identifying the products, determining the type of reaction, and applying the principles of ionic pairing to predict the formation of magnesium nitrate and silver chloride. The paragraph also discusses the importance of knowing the charges of ions and the solubility rules to identify precipitates and balance the chemical equation. Finally, it explains how to write the total ionic equation and identify spectator ions to derive the net ionic equation for the reaction.
🧪 Acid-Base Reactions and Solubility Rules
The second paragraph delves into acid-base reactions, using the reaction between sulfuric acid and aqueous sodium hydroxide as an example. It explains that these reactions are typically double replacement reactions and demonstrates how to pair ions to form water and sodium sulfate. The paragraph emphasizes the solubility of group one metal cations and polyatomic ions like sulfate. It also guides on balancing the reaction and writing the total ionic equation, highlighting the need to recognize and eliminate spectator ions to obtain the net ionic equation. The discussion extends to the reaction between a weak acid (HF) and a strong base (KOH), noting the difference in ionization between weak acids and strong acids in the context of writing total ionic equations.
🌌 Gas Evolution Reactions and Solubility Exceptions
This paragraph focuses on gas evolution reactions, exemplified by the reaction between aqueous sodium carbonate and hydrochloric acid. It outlines the process of identifying the products, which include water and carbon dioxide gas, and emphasizes the importance of knowing the solubility rules for various ions. The paragraph explains how to write the total ionic equation, remove spectator ions, and obtain the net ionic equation for the reaction. It also reviews solubility rules, highlighting exceptions for halides, sulfates, hydroxides, carbonates, and other ions, and provides a brief overview of another gas evolution reaction involving ammonium chloride and strontium hydroxide.
🔋 Activity Series and Single Replacement Reactions
The fourth paragraph introduces the concept of the activity series, which ranks metals and nonmetals by their reactivity. It explains that a more reactive metal can displace a less reactive metal from its compound in a single replacement reaction. Using the reaction between aluminum metal and copper chloride as an example, the paragraph demonstrates how to predict products, balance the equation, and write the total ionic and net ionic equations. It also discusses why certain reactions, like the one between nickel metal and iron sulfate, do not occur based on the reactivity order. The paragraph concludes with an example of a nonmetal displacement reaction where chlorine gas reacts with aqueous sodium bromide, resulting in the formation of sodium chloride and bromine.
🌟 Writing Net Ionic Equations for Single Replacement Reactions
The fifth paragraph provides a detailed explanation of how to write net ionic equations for single replacement reactions. It emphasizes the role of the activity series in determining the feasibility of such reactions. The paragraph illustrates the process with the reaction between aluminum and copper chloride, showing how to identify the products, balance the chemical equation, and derive the net ionic equation by recognizing and eliminating spectator ions. It also addresses a scenario where nickel metal does not react with iron sulfate due to the reactivity order, and a chlorine gas reaction with aqueous sodium bromide, resulting in the formation of sodium chloride and bromine, with the net ionic equation reflecting the aqueous phase of the reactants and products.
🌠 Double Replacement Precipitation Reactions
The sixth paragraph concludes the video script with an example of a double replacement precipitation reaction between aqueous calcium nitrate and sodium phosphate. It guides the viewer through predicting the products, ensuring the reaction is balanced, and writing the total ionic equation. The paragraph explains the identification of spectator ions, which are nitrate and sodium in this case, and derives the net ionic equation by focusing on the ions that participate in the reaction to form the precipitate, calcium phosphate. The video aims to enhance the viewer's understanding of writing net ionic equations across different types of chemical reactions.
Mindmap
Keywords
💡Double Replacement Reaction
💡Precipitation Reaction
💡Solubility Rules
💡Net Ionic Equation
💡Spectator Ions
💡Activity Series
💡Single Replacement Reaction
💡Gas Evolution Reaction
💡Acidity and Basicity
💡Polyatomic Ions
💡Criscross Method
Highlights
The video focuses on writing net ionic equations for various types of chemical reactions including double replacement, precipitation, acid-base, gas evolution, and single replacement reactions.
Double replacement reactions are identified by the form A B + C D, where A and D combine to form AD, and B and C combine to form BC.
Magnesium chloride and silver nitrate undergo a double replacement reaction to form magnesium nitrate and silver chloride, with silver chloride being insoluble and forming a precipitate.
The charges of ions are crucial in writing formulas using the crisscross method, especially for polyatomic ions like nitrate.
Solubility rules are essential for determining the phases of substances in a reaction, such as whether a compound is soluble or will form a precipitate.
Acid-base reactions, typically double replacement reactions, involve the formation of water from H+ and OH- ions.
Total ionic equations separate substances in the aqueous phase into ions, while net ionic equations eliminate spectator ions that remain unchanged.
Weak acids like HF do not fully ionize, so they should not be separated into ions when writing total ionic equations.
Gas evolution reactions, such as the reaction between sodium carbonate and hydrochloric acid, produce a gas, carbon dioxide, along with water.
The activity series is a guide to predict whether a single replacement reaction will occur based on the reactivity of metals.
Aluminum, being higher in the activity series, can displace copper from copper chloride solution, forming aluminum chloride and solid copper.
Nickel, lower in the activity series than iron, cannot displace iron from iron sulfate solution, resulting in no reaction.
Chlorine gas, being more reactive than bromine, can displace bromine from sodium bromide, forming sodium chloride and liquid bromine.
Double replacement precipitation reactions, such as the reaction between calcium nitrate and sodium phosphate, result in the formation of an insoluble solid, calcium phosphate.
Balancing chemical equations involves ensuring the number of atoms for each element is the same on both sides of the equation.
Writing the total ionic equation involves separating all aqueous substances into their respective ions.
Net ionic equations are derived from total ionic equations by removing the spectator ions, showing only the ions that participate in the reaction.
Understanding solubility rules and the reactivity of different ions and metals is key to predicting the products of chemical reactions and writing correct ionic equations.
Transcripts
Browse More Related Video

Chemical Reactions

Classifying Types of Chemical Reactions Practice Problems

Introduction to Double Replacement Reactions

Chemical Reactions - Combination, Decomposition, Combustion, Single & Double Displacement Chemistry

Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems
Chemical Reactions (2 of 11) Single Replacement Reactions, An Explanation
5.0 / 5 (0 votes)
Thanks for rating: