12.31 | The following data have been determined for the reaction: I− + OCl− → IO− + Cl−
TLDRIn this educational video, the presenter guides viewers through the process of determining the rate law and rate constant for a chemical reaction involving I- and OCl-. The script explains the concept of rate laws, the importance of identifying reaction orders, and demonstrates how to use algebraic ratios to solve for these orders using given experimental data. The presenter simplifies complex concepts with humor and patience, ensuring a clear understanding of the methodology for finding the rate constant and its units, ultimately aiming to assist students in grasping key chemistry principles.
Takeaways
- 🧪 The video discusses determining the rate law and rate constant for a chemical reaction from a given set of trials.
- 📊 It emphasizes the importance of understanding the generalized rate law formula: rate = k * [reactants]^orders.
- 🔍 The process involves analyzing trials with varying concentrations of reactants to deduce the reaction's order.
- 📐 The video suggests using algebra to solve for the orders of the reactants by creating ratios of rate laws.
- 📝 It highlights the need to identify trials where one reactant's concentration remains constant to isolate the variable of interest.
- ✅ The script demonstrates solving for the order of one reactant (I^-) by comparing trials with the same OCl^- concentration.
- ✏️ After determining the order for one reactant, the process is repeated to find the order for the other reactant (OCl^-).
- 🔢 The video provides a step-by-step guide on how to perform the calculations, including dividing rate values and concentrations.
- 📉 It explains that if certain trials with identical reactant concentrations are not provided, a more complex mathematical approach is necessary.
- 📚 The final rate law is presented once the orders for both reactants are found, and the rate constant is calculated using the values from a specific trial.
- 🔑 The units of the rate constant are linked to the overall order of the reaction, which is the sum of the individual orders of the reactants.
- 🌟 The video concludes by expressing gratitude to the viewers and mentioning the channel's plans to expand content to different subjects.
Q & A
What is the reaction given in the script?
-The reaction given in the script is I^- + OCl^- → IO^- + Cl^-.
What is the purpose of analyzing the chart in the script?
-The purpose of analyzing the chart is to determine the rate law and the rate constant for the given chemical reaction based on the trials conducted with different concentrations of I^- and OCl^-.
What is the generalized rate law formula mentioned in the script?
-The generalized rate law formula mentioned in the script is rate = k * [reactant]^n, where k is the rate constant, [reactant] is the concentration of the reactant, and n is the order of the reaction.
Why is it important to find the orders of the reactants in the rate law?
-Finding the orders of the reactants is important because it helps in writing the specific rate law for the reaction, which is essential for understanding how the rate of the reaction is affected by the concentrations of the reactants.
How does the script suggest solving for the orders of the reactants?
-The script suggests solving for the orders by using algebraic ratios of the rate laws from different trials, where the concentrations of one reactant are held constant to isolate the variable for the other reactant.
What is the significance of the trials having different concentrations of reactants?
-The different concentrations in the trials allow for the determination of how changes in concentration affect the reaction rate, which is crucial for finding the reaction orders.
What is the rate law found for the reaction in the script?
-The specific rate law found for the reaction in the script is rate = k * [I^-]^1 * [OCl^-]^1, indicating that both reactants have an order of 1.
How is the rate constant (k) determined from the trials?
-The rate constant (k) is determined by using the rate law and the known concentrations of the reactants from a trial, solving for k when the rate is known.
What is the overall order of the reaction based on the rate law found?
-The overall order of the reaction is 2, as the sum of the orders of the reactants (1 for I^- and 1 for OCl^-) is 2.
What are the units of the rate constant (k) for a second-order reaction?
-For a second-order reaction, the units of the rate constant (k) are typically in terms of concentration to the power of -1 and time to the power of -1, for example, M^-1*s^-1 if molarity and seconds are used.
Outlines
🔍 Introduction to Determining Rate Law and Constant
The script begins with an introduction to a chemistry experiment involving the reaction of I⁻ and OCl⁻ to form IO⁻ and Cl⁻. The presenter discusses the need to determine the rate law and rate constant from a provided chart, which includes data from three trials with varying concentrations of the reactants. The emphasis is on understanding the relationship between reactant concentrations and reaction rates, and the importance of identifying the reaction orders from the data.
📚 Methodology for Finding Reaction Orders
This paragraph delves into the process of finding the reaction orders by using algebraic ratios derived from the experimental data. The presenter explains the strategy of selecting trials with constant concentrations of one reactant to isolate and solve for the order of the other. The method involves creating a ratio of rate laws and simplifying it to solve for the exponents (orders) of the reactants in the rate law expression.
🔢 Solving for the Order of I⁻ Using Trial Data
The presenter demonstrates the step-by-step process of solving for the order of I⁻ by comparing trials with the same concentration of OCl⁻. Using the rate values from these trials, a ratio is formed, and algebra is used to determine the exponent for I⁻. The calculations lead to the discovery that the order of I⁻ is 1, as the rate is directly proportional to its concentration.
🔄 Attempt to Solve for the Order of OCl⁻ and its Challenges
In this section, the presenter attempts to solve for the order of OCl⁻ but encounters a challenge due to the lack of trials with constant I⁻ concentrations. Despite this, the presenter explains that it's still possible to determine the order by using the information from any two trials and performing additional algebraic steps. The presenter guides through the calculations, which eventually lead to determining the order of OCl⁻.
🎯 Finalizing the Rate Law and Calculating the Rate Constant
After determining the orders of both reactants, the presenter finalizes the rate law expression. The rate constant is then calculated using the values from one of the trials. The presenter emphasizes the importance of understanding the units of the rate constant, which are related to the overall order of the reaction. The rate constant is found to be 0.061, with units of M⁻¹s⁻¹, given the second-order reaction.
🌟 Conclusion and Appreciation for the Audience
The script concludes with the presenter expressing gratitude to the audience for their engagement and support. They mention the duration of the video in a humorous way, reflecting on the effort put into creating educational content. The presenter also hints at future plans for the channel, including expanding to different subjects and providing more educational resources.
Mindmap
Keywords
💡Rate Law
💡Rate Constant (k)
💡Reactants
💡Concentration
💡Trials
💡Orders of Reaction
💡Generalized Rate Law
💡Algebra
💡Molarity
💡Overall Order
💡Units of K
Highlights
Introduction to the process of determining the rate law and rate constant for a chemical reaction.
Explanation of the generalized rate law formula and its significance in finding reaction orders.
Description of the experimental setup involving different trials with varying concentrations of reactants.
The importance of identifying the orders of reactants from the rate law formula.
Demonstration of using algebra to solve for the reaction orders by comparing different trials.
Strategy for selecting trials to isolate and solve for individual reactant orders.
Calculation of the rate law using specific trial data and the cancellation of constants.
Determination of the order for I- (Iodide) through a ratio comparison of two trials.
Finding the order for OCl- (Oxidized Chloride) by comparing trials with different concentrations.
Use of trial data to calculate the rate constant for the reaction.
Discussion on the units of the rate constant and their relation to the overall reaction order.
Explanation of the overall reaction order and how it sums up the individual orders of reactants.
Finalization of the specific rate law formula with identified orders and constants.
Practical application of the rate law in understanding and predicting reaction rates.
Engagement with the audience, encouraging them to try different trials to verify the consistency of the rate constant.
Reflection on the educational value of the process and its relevance to students' learning.
Closing remarks, expressing gratitude to the audience and highlighting the channel's educational mission.
Transcripts
Browse More Related Video
14.2 Rate Laws | General Chemistry
12.33 | Use the data provided to graphically determine the order and rate constant of the following
Units of the rate constant | Kinetics | AP Chemistry | Khan Academy
12.34 | Pure ozone decomposes slowly to oxygen, 2O3(g) → 3O2(g). Use the data provided in a
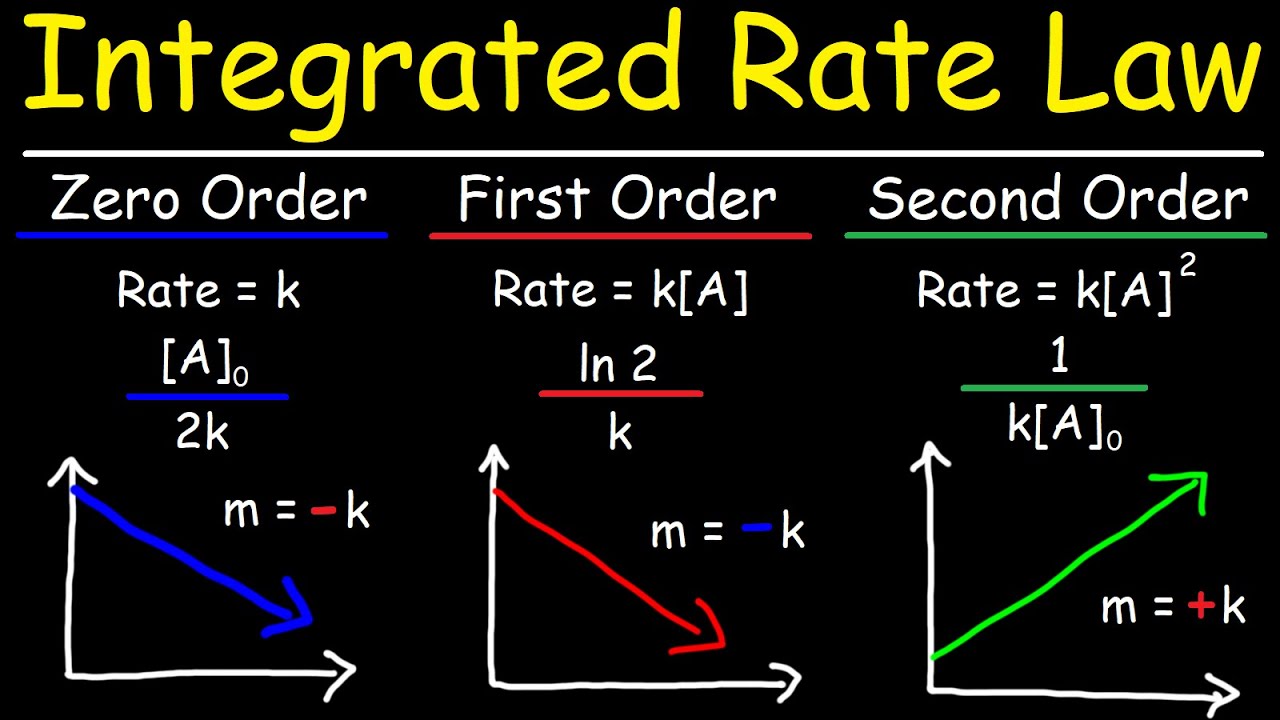
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics

AP Chem Unit 5 Review - Kinetics in 10 Minutes!
5.0 / 5 (0 votes)
Thanks for rating: