14.2 Rate Laws | General Chemistry
TLDRIn this chemistry lesson, Chad from Chad's Prep simplifies the concept of rate laws, guiding students through understanding rate constants, reactant orders, and overall reaction order. He emphasizes the importance of deriving rate laws from experimental data, a common challenge for students. Chad explains the significance of zero, first, and second order reactions, illustrating how concentration changes affect reaction rates. He uses an analogy of diagnosing a car problem to demonstrate the method of isolating variables in experiments. Through analyzing data from a reaction between NO and Cl2, Chad shows how to determine the reaction's order and calculate the rate constant, ultimately enabling predictions of reaction rates without further experiments.
Takeaways
- 📚 The lesson focuses on understanding rate laws, rate constants, reactant orders, and determining the rate law from experimental data in chemistry.
- 🔍 Chad's Prep aims to simplify the learning process for high school and college science, as well as DAT, MCAT, and OAT prep.
- 📈 A rate law predicts the rate of a chemical reaction, similar to how the law of gravity predicts the fall of an object when released.
- 📝 The general form of a rate law includes a rate constant (k), reactant concentrations, and their respective exponents (orders).
- 🧬 Reactant orders are typically integers and can be zero, one, or two, but they are not always equal to the coefficients in a balanced chemical equation.
- 🔢 The overall reaction order is the sum of the individual reactant orders and can be determined through experimental data.
- 🚫 It's a common misconception that reactant orders are always integers; they can also be fractions or decimals in some cases.
- 🌡 The rate constant (k) is a proportionality constant that may change with temperature but remains constant for a given reaction at a constant temperature.
- 🧪 Experimental data is crucial for determining the rate law, as it provides the evidence needed to ascertain the orders of reactants.
- 📉 When analyzing experimental data, changing one variable at a time helps isolate the effect of each reactant on the reaction rate.
- 📚 The script provides an example of determining the rate law for the reaction between NO and Cl2 to form NOCl, illustrating how to deduce reactant orders and calculate the rate constant.
Q & A
What is the main topic of Chad's lesson?
-The main topic of Chad's lesson is rate laws, including rate constants, reactant orders, and determining the rate law of a reaction based on experimental data.
What is the significance of a rate law in chemistry?
-A rate law in chemistry allows us to make predictions about the rate of a chemical reaction, similar to how the law of gravity allows us to predict the behavior of objects in free fall.
What is the difference between a lowercase 'k' and an uppercase 'K' in the context of rate laws?
-In the context of rate laws, a lowercase 'k' represents the rate constant, which is a proportionality constant. An uppercase 'K' is used for other purposes, such as the equilibrium constant, and should not be confused with the rate constant.
What are the exponents in a rate law called, and what do they represent?
-The exponents in a rate law are called orders, and they represent the relationship between the concentration of reactants and the rate of the reaction.
What is the overall reaction order, and how is it determined?
-The overall reaction order is the sum of all the individual reactant orders. It is determined by adding up the exponents of the reactant concentrations in the rate law.
Why can't the reaction orders be determined by just looking at the balanced chemical equation?
-The reaction orders cannot be determined by just looking at the balanced chemical equation because the exponents in the rate law do not necessarily correspond to the stoichiometric coefficients in the balanced equation. They must be determined experimentally.
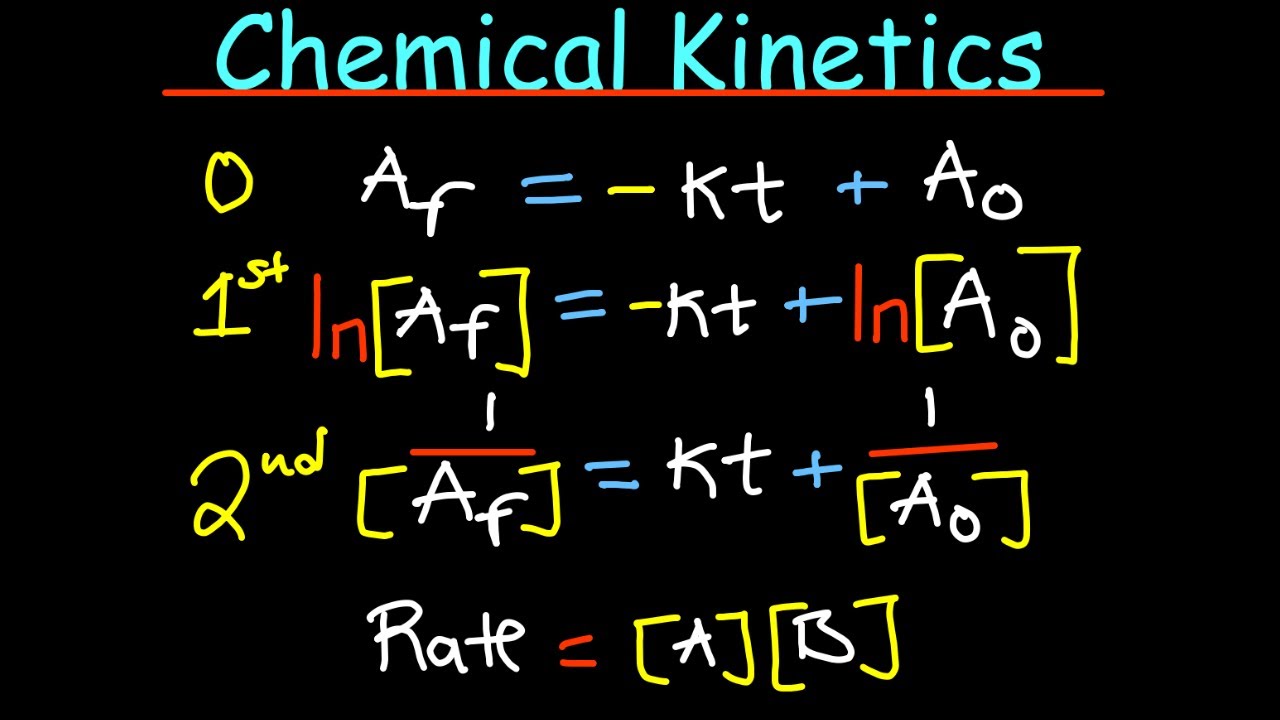
What is a zero-order reaction with respect to a reactant, and how does it affect the reaction rate?
-A zero-order reaction with respect to a reactant means that the concentration of that reactant does not affect the rate of the reaction. The rate remains constant regardless of the concentration of the zero-order reactant.
What is the hallmark of a first-order reaction, and how does it relate to the concentration of the reactant?
-The hallmark of a first-order reaction is that the rate of the reaction is directly proportional to the concentration of the reactant. If the concentration of the reactant is doubled, the rate of the reaction also doubles.
How does a second-order reaction differ from a first-order reaction in terms of the relationship between concentration and reaction rate?
-In a second-order reaction, the rate is proportional to the square of the concentration of the reactant. If the concentration is doubled, the rate increases by a factor of four, which is the square of two.
What is the process of determining the rate law from experimental data, and why is it important?
-Determining the rate law from experimental data involves comparing the changes in reaction rate with changes in reactant concentrations to identify the reaction orders. This process is important because it allows us to predict the rate of a reaction without having to perform the reaction in the lab, and it provides insights into the reaction mechanism.
Outlines
🔬 Introduction to Rate Laws and Experimental Determination
Chad introduces the topic of rate laws, emphasizing the importance of understanding rate constants, reactant orders, and the overall reaction order. He assures that despite the common struggle students face, the lesson will simplify the concept. Chad's Prep aims to ease the learning of science, offering high school, college, and DAT, MCAT, and OAT prep. The lesson is part of a general chemistry playlist with regular uploads. Chad explains the rate law's predictive nature, using the law of gravity as an analogy, and introduces the components of a rate law, including the rate constant (lowercase k), reactant concentrations, and the significance of the exponents (orders). He clarifies that orders are not always integers and are derived from experimental data rather than reaction coefficients.
🧪 Understanding Reactant Orders and Their Impact on Reaction Rates
This paragraph delves into the specifics of reactant orders, explaining that they are often integers and most frequently zero, one, or two. Chad discusses the concept of zero-order reactions, where reactant concentration does not affect the rate, using the formula k[A]^0 to illustrate that the rate remains constant regardless of [A]'s concentration. He contrasts this with first-order reactions, where the rate is directly proportional to the concentration of the reactant, exemplified by doubling the concentration of M leading to a doubling of the reaction rate. Chad also introduces second-order reactions, where the rate is proportional to the square of the concentration, meaning that doubling the concentration of X results in a quadrupling of the rate. He emphasizes the importance of experimental data in determining these orders.
🔍 Analyzing Experimental Data to Determine Reaction Orders
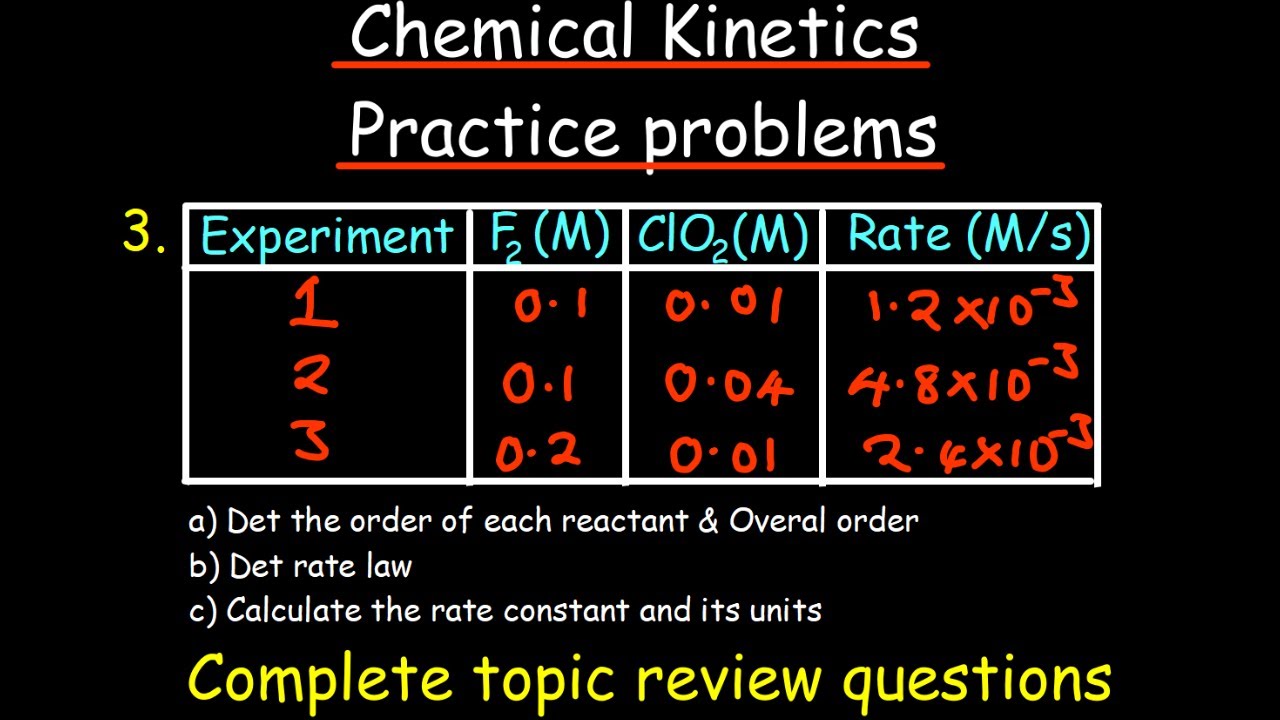
Chad presents an analogy of diagnosing a vehicle problem to explain the method of changing one variable at a time to identify the cause of changes in reaction rates. He applies this principle to experimental data involving the reaction between NO and Cl2 to form NOCl. By comparing trials with different concentrations of NO and Cl2, Chad demonstrates how to deduce the reaction orders. He explains that by observing the changes in reaction rates in response to changes in reactant concentrations, one can determine the order of the reactants. The method involves comparing the rates and concentrations from different trials to find a consistent pattern that matches the expected outcomes for zero, first, or second-order reactions.
📚 Calculating the Rate Constant from Determined Reaction Orders
Having established the reaction orders from experimental data, Chad proceeds to calculate the rate constant (k). He uses the formula k = rate / ([NO]^2 * [Cl2]), plugging in the values from the first trial to find k's value. Chad highlights the importance of keeping track of units throughout the calculation, noting that the units on the rate constant can reveal the overall reaction order. He explains that the units of k will correspond to the inverse of the overall reaction order, providing a method to verify the determined orders without direct calculation.
📉 Writing the Overall Rate Law and Predicting Reaction Rates
With the reaction orders and the rate constant determined, Chad writes the overall rate law for the reaction between NO and Cl2. He emphasizes that the rate law allows for the prediction of reaction rates without the need for further experimentation. Chad demonstrates how to use the rate law to calculate the rate of the reaction given different initial concentrations of reactants. He also notes that while the coefficients in a balanced chemical equation may sometimes correspond to the reaction orders, this is not a rule and must be verified through experimental data.
👋 Conclusion and Additional Resources for Kinetics Practice
In conclusion, Chad invites feedback on the lesson and promotes his general chemistry master course for further practice in kinetics and determining rate laws from experimental data. The course includes over 1,200 practice questions and video solutions, with a free trial available. Chad encourages students to use the knowledge gained from the lesson to predict reaction rates and to seek additional help if needed.
Mindmap
Keywords
💡Rate Law
💡Rate Constant (k)
💡Reactant Orders
💡Overall Reaction Order
💡Zero Order Reaction
💡First Order Reaction
💡Second Order Reaction
💡Experimental Data
💡Concentration
💡Units of Rate Constant
Highlights
Introduction to the concept of rate laws and their importance in predicting reaction rates based on experimental data.
Explanation of rate constants (lowercase k) and their role as proportionality constants in rate laws.
Discussion on reactant orders and how they are typically represented as exponents in rate laws.
Clarification that reactant orders are not always integers and can be fractions or decimals in certain experimental conditions.
Illustration of zero-order reactions with a practical example showing concentration does not affect the reaction rate.
Description of first-order reactions, emphasizing how the reaction rate is directly proportional to the concentration of the reactant.
Analysis of second-order reactions, where the rate is proportional to the square of the reactant concentration.
Introduction of an analogy comparing diagnosing vehicle problems to determining reaction orders from experimental data.
Demonstration of how to examine experimental data to deduce the order of reactants in a reaction.
Use of a step-by-step example to show the process of determining the rate law for a reaction between NO and Cl2.
Explanation of how to calculate the rate constant (k) using known rates and concentrations from experimental trials.
Insight into the relationship between the units of the rate constant and the overall reaction order.
Highlighting the ability to predict reaction rates without conducting experiments once the rate law is established.
Note on the potential discrepancy between the coefficients in a balanced chemical equation and the actual reaction orders.
Encouragement for students to engage with the content by providing feedback in the comments section.
Promotion of the General Chemistry Master Course for additional practice and understanding of kinetics and rate laws.
Transcripts
Browse More Related Video

AP Chem Unit 5 Review - Kinetics in 10 Minutes!
Chemical Kinetics practice problems - complete review

15.1 Chemical Equilibrium and Equilibrium Constants | General Chemistry

Concentration Changes Over Time - AP Chem Unit 5, Topic 3
Chemical Kinetics summary in 30 minutes

12.32 | Describe how graphical methods can be used to determine the order of a reaction and its rate
5.0 / 5 (0 votes)
Thanks for rating: