Units of the rate constant | Kinetics | AP Chemistry | Khan Academy
TLDRThis educational video script explains the concept of rate constants in chemical reactions, emphasizing that the units of the rate constant 'k' are dependent on the rate law of the reaction. It demonstrates how to derive the units for zeroth, first, and second-order reactions by using the rate law, ensuring the units on both sides of the equation match. The script simplifies the process by showing that units can be treated like numbers, which must balance out in any equation, providing a clear method to understand the units of 'k' without memorization.
Takeaways
- 🔍 The rate constant 'k' in chemical reactions has units and these units are dependent on the rate law of the reaction.
- 📚 Understanding the rate law is essential to determine the units of 'k' without needing to memorize them for different reaction orders.
- 🧪 The three common reaction orders discussed are zeroth, first, and second order reactions, each with a unique rate law and corresponding units for 'k'.
- ⏱ For zeroth order reactions, the rate is equal to 'k', and since the rate's units are molar per second, 'k' must also be in molar per second.
- 📉 In first order reactions, the rate law is rate = k[A], where [A] is the concentration of reactant A. The units of 'k' are derived to be one over seconds (s⁻¹).
- 🔄 The units of 'k' can be found by rearranging the rate law equation and dividing the units of rate by the units of concentration.
- 📈 For second order reactions, the rate law is rate = k[A]², leading to 'k' having units of one over molar times seconds (M⁻¹s⁻¹).
- 📘 The concept of balancing units is crucial; units must match on both sides of an equation, similar to how numbers must balance in an equation.
- 🔎 By understanding the relationship between the units of rate, concentration, and 'k', one can deduce the units of 'k' for any reaction order.
- 📝 The script provides a methodical approach to derive the units of 'k' using the rate law, which is a practical tool for students in chemistry.
- 🌐 This understanding is applicable not only to the most common reaction orders but also to any reaction where the order is not zeroth, first, or second, by using the rate law to determine 'k's units.
Q & A
What is the significance of the rate constant k in chemical reactions?
-The rate constant k is significant because it determines the speed at which a chemical reaction proceeds. It is a measure of how quickly reactants are converted into products.
Why do the units of the rate constant k matter in chemistry?
-The units of k are crucial because they ensure the consistency of units in the rate law equation. They help in correctly calculating the reaction rate and understanding the reaction kinetics.
How does the rate law determine the units of the rate constant k?
-The units of k depend on the rate law of the reaction. By analyzing the rate law, you can derive the appropriate units for k, ensuring that the units on both sides of the rate equation balance.
What are the three common orders of reactions discussed in the script?
-The script discusses zeroth, first, and second order reactions, which are the most common types of reactions in chemistry.
What is the rate law for a zeroth order reaction, and what are its units?
-The rate law for a zeroth order reaction is rate = k. Since the concentration of the reactant is raised to the zeroth power, the rate is simply equal to the rate constant k. The units for k in a zeroth order reaction are moles per second (M/s).
How can you determine the units of k for a first order reaction?
-For a first order reaction, the rate law is rate = k[A], where [A] is the concentration of reactant A. Since the units of rate are moles per second (M/s) and the units of concentration are moles per liter (M), the units of k must be seconds to the power of -1 (s^-1) to balance the equation.
What rearrangement of the rate law can help determine the units of k for a first order reaction?
-You can rearrange the rate law to k = rate / [A]. This shows that k is the rate divided by the concentration, and by dividing the units of rate (M/s) by the units of concentration (M), you get the units of k as seconds to the power of -1 (s^-1).
What is the rate law for a second order reaction, and what are its units?
-The rate law for a second order reaction is rate = k[A]^2. Here, the concentration of reactant A is squared. The units of k for a second order reaction are moles per liter-second (M/(L·s)) to balance the units on both sides of the equation.
How can you ensure that the units of k are correct for a second order reaction?
-By ensuring that the units of k, when multiplied by the squared concentration (M^2), result in the units of rate (M/s). This means k must have units of moles per liter-second (M/(L·s)) to cancel out the concentration terms correctly.
What is a general approach to finding the units of k for reactions that are not zeroth, first, or second order?
-For reactions that do not follow the zeroth, first, or second order kinetics, you can always use the rate law equation to derive the units of k. By balancing the units on both sides of the equation, you can determine the appropriate units for k.
Outlines
🔍 Understanding Rate Constant Units
This paragraph introduces the concept of rate constants in chemical reactions, emphasizing that they have units and these units depend on the rate law. The speaker explains that the process of deriving the units of k from the rate law eliminates the need to memorize units for different reaction orders. The video focuses on deriving the units for zeroth, first, and second order reactions, starting with zeroth order, where the rate is equal to k and the units are molar per second.
📚 Deriving Units for First and Second Order Reactions
The second paragraph continues the discussion on rate constants, specifically addressing first and second order reactions. For first order reactions, the rate law is presented, and it's explained that the units of k must be one over seconds to balance the units on both sides of the equation. The speaker also suggests rearranging the rate law to isolate k and then determining its units by dividing the units of rate by the units of concentration. For second order reactions, the rate law involves the concentration squared, leading to units of k being one over molar times seconds. The paragraph concludes with a reminder that for reactions not fitting the zeroth, first, or second order, the rate law can still be used to determine the units of k.
Mindmap
Keywords
💡Rate Constant
💡Units
💡Rate Law
💡Zeroth Order Reaction
💡First Order Reaction
💡Second Order Reaction
💡Concentration
💡Molar
💡Reaction Order
💡Derive
💡Balance Equation
Highlights
The video discusses how to find the units for the rate constant k in chemical reactions.
Rate constant k has units, which is not always the case for constants in chemistry.
The units of k depend on the rate law of the reaction.
Deriving the units of k from the rate law eliminates the need to memorize units for different reaction orders.
The video focuses on zeroth, first, and second-order reactions to derive their units.
For zeroth-order reactions, the rate law is rate = k, indicating k has units of molar per second.
The units of rate are always molar per second, regardless of the reaction order.
Units must match on both sides of an equation, which helps in determining the units of k.
For first-order reactions, the rate law is rate = k * concentration, leading to k having units of one over seconds.
Rearranging the rate law for first-order reactions helps in finding the units of k by division.
The units of k for first-order reactions are one over seconds, derived by dividing units of rate by concentration units.
Second-order reactions have a rate law of rate = k * concentration squared.
For second-order reactions, k's units are one over molar times seconds, derived from the rate law.
The video demonstrates the method to find units of k by treating units as numbers and ensuring they match on both sides of the equation.
Non-standard reaction orders can also have their k units determined using the rate law.
The video provides a practical approach to understanding the relationship between rate laws and units of k.
Understanding the units of k is crucial for correctly interpreting and calculating chemical reaction rates.
Transcripts
Browse More Related Video
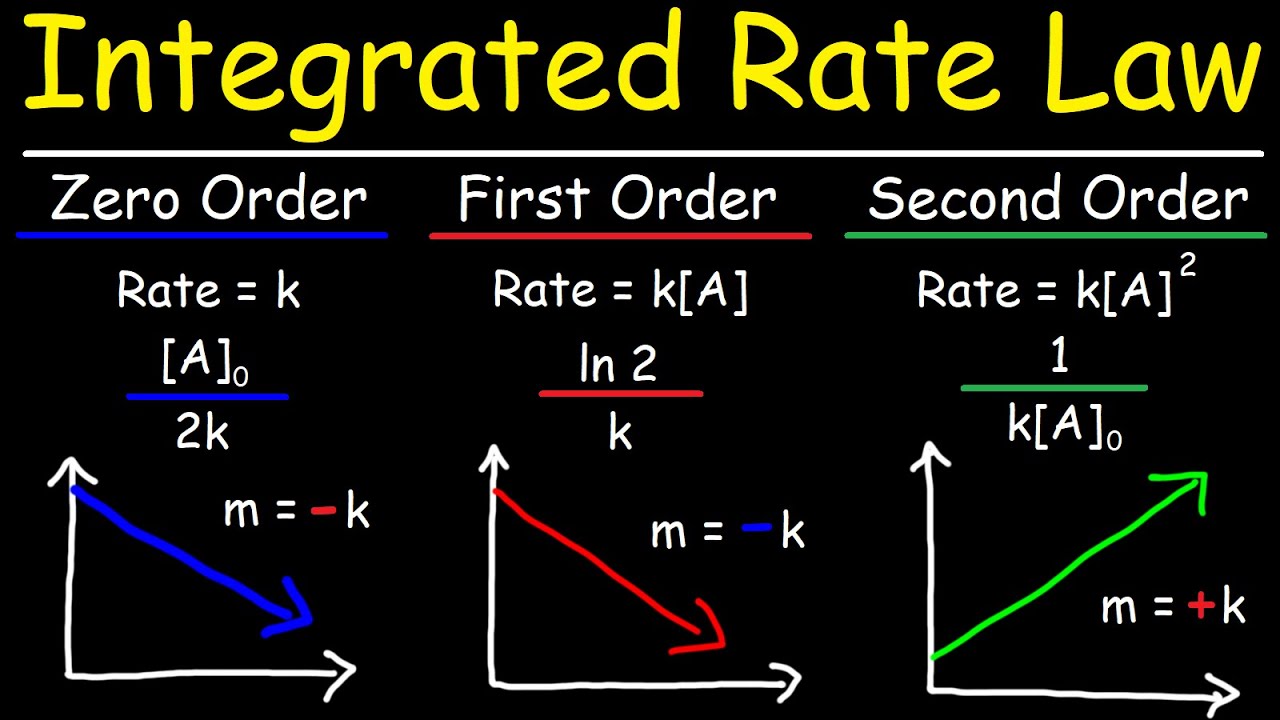
Integrated Rate Laws - Zero, First, & Second Order Reactions - Chemical Kinetics

Rate constant k from half-life example | Kinetics | Chemistry | Khan Academy

12.31 | The following data have been determined for the reaction: I− + OCl− → IO− + Cl−
12.33 | Use the data provided to graphically determine the order and rate constant of the following

Arrhenius Equation Activation Energy and Rate Constant K Explained

12.32 | Describe how graphical methods can be used to determine the order of a reaction and its rate
5.0 / 5 (0 votes)
Thanks for rating: