Steam tables: example 1
TLDRThis instructional transcript guides viewers on calculating thermodynamic properties of water, emphasizing the importance of understanding phase diagrams for analyzing steam power plants and air-conditioning systems. It demonstrates the process of finding the internal energy of superheated water at 240°C and 2000 kPa by referencing the saturation dome on a temperature-volume diagram, consulting the saturated water table, and using the superheated vapor table at the exact pressure and temperature to determine the specific internal energy as 2659.6 kJ/kg.
Takeaways
- 📚 The video is focused on calculating thermodynamic properties of water, which is crucial for analyzing steam power plants and air-conditioning systems.
- 📈 It is recommended to draw a phase diagram to visualize and understand the state of water in different phases.
- 🌡️ The example problem involves finding the internal energy of water at 240°C and 2000 kPa.
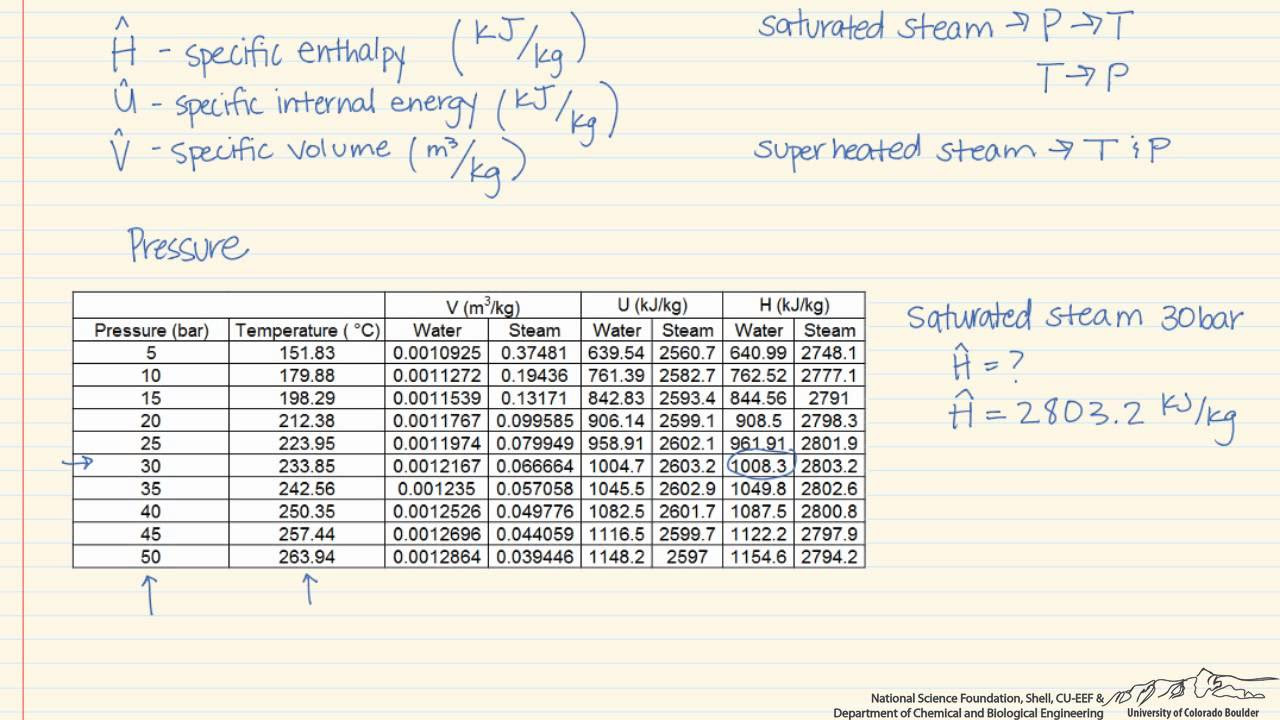
- 🔍 The saturation water table is used to determine the saturation pressure at a given temperature, which in this case is 240°C.
- 💧 The saturation pressure at 240°C is found to be 33.44 bar, which is equivalent to 3344 kPa.
- 📊 A temperature-volume (T-V) diagram is used to map out different phases and regions, with temperature on the vertical axis and specific volume on the horizontal axis.
- 📉 The constant pressure curve at the saturation pressure (3344 kPa) is drawn on the T-V diagram.
- 🔑 The actual pressure of the problem (2000 kPa) is less than the saturation pressure, indicating the state is in the superheated vapor region.
- 🌐 The state of water is determined to be at the intersection of the constant pressure curve for 2000 kPa and the temperature line at 240°C.
- 🔍 The superheated vapor table is used to find the specific internal energy (u) at the given conditions, which is found to be 2659.6 kJ/kg.
Q & A
What is the main topic of the transcript?
-The main topic of the transcript is the calculation of thermodynamic properties of water, specifically finding the internal energy of water at a given temperature and pressure.
Why is it important to understand thermodynamic properties of water in this context?
-Understanding thermodynamic properties of water is crucial for analyzing steam power plants, air-conditioning systems, and any other processes involving water in liquid or vapor states.
What is the first step suggested for solving problems related to thermodynamic properties of water?
-The first step suggested is to draw a phase diagram, which helps in visualizing and understanding the state of water.
What are the axes of the temperature-volume (TV) phase diagram mentioned in the transcript?
-The temperature is on the vertical axis, and the specific volume is on the horizontal axis of the TV phase diagram.
What is the significance of the saturation dome in the phase diagram?
-The saturation dome in the phase diagram represents the region where water can exist as a liquid-vapor mixture and helps map out different phases and regions of water.
What tables are referred to as 'saturated water tables' in the transcript?
-The 'saturated water tables' refer to the superheated water table and the saturated water pressure table, which are used to determine properties at saturation conditions.
What is the saturation pressure of water at 240 degrees Celsius according to the transcript?
-The saturation pressure of water at 240 degrees Celsius is 33.44 bar, which is equivalent to 3344 kiloPascals.
Why does the pressure being less than the saturation pressure indicate superheated vapor?
-If the pressure is less than the saturation pressure at a given temperature, it indicates that the water is in a superheated vapor state, not in a liquid or liquid-vapor mixture state.
What table should be used to find the internal energy of superheated vapor?
-The superheated vapor table should be used to find the internal energy of superheated vapor at a specific temperature and pressure.
What is the specific internal energy of water at 240 degrees Celsius and 2000 kiloPascals according to the transcript?
-The specific internal energy of water at 240 degrees Celsius and 2000 kiloPascals is 2659.6 kilojoules per kilogram.
What is the importance of drawing a phase diagram in understanding the state of water in the given problem?
-Drawing a phase diagram helps in visualizing the state of water and determining whether it is in a superheated vapor, liquid, or liquid-vapor mixture state, which is essential for selecting the correct table for calculations.
Outlines
🔍 Thermodynamic Properties of Water Calculation
This paragraph introduces a series of worked examples on calculating the thermodynamic properties of water, which is essential for analyzing steam power plants, air-conditioning systems, and any processes involving water in liquid or vapor states. The first example focuses on finding the internal energy of water at 240°C and 2000 kPa. The speaker advises drawing a phase diagram, specifically a temperature-volume diagram, to visualize the state of water. The process involves consulting the saturated water table to determine the saturation pressure at the given temperature, which is found to be 33.44 bar or 3344 kPa. The diagram helps to identify the state of water as superheated vapor because the given pressure is less than the saturation pressure. The state is graphically determined at the intersection of the constant temperature and constant pressure curves on the phase diagram.
📚 Locating Superheated Vapor Properties in Tables
The second paragraph continues the discussion on identifying the state of water as superheated vapor and emphasizes the importance of understanding this to select the correct table for further calculations. The speaker explains that since the pressure is below the saturation pressure, the state is confirmed as superheated vapor. The process then involves referring to the superheated vapor table, which is organized by pressure. Fortunately, there is a specific table for the exact pressure of 2000 kPa (2 MPa), and within this table, a row for 240°C is available. The internal energy (u) of the superheated vapor at these conditions is found to be 2659.6 kJ/kg, which is the specific internal energy value needed for the analysis. The summary concludes with the final answer, including the units, providing a clear and concise result for the example problem.
Mindmap
Keywords
💡Thermodynamic properties
💡Phase diagram
💡Saturated water table
💡Saturation pressure
💡Specific volume
💡Superheated vapor
💡Constant pressure curve
💡Internal energy
💡Kilojoules per kilogram
💡Superheated vapor table
Highlights
Introduction to calculating thermodynamic properties of water, essential for analyzing steam power plants and air-conditioning systems.
Advice to draw a phase diagram to visualize and understand the state of water in liquid or vapor form.
Familiarity with temperature-volume (TV) diagrams for thermodynamics, using temperature on the vertical axis and specific volume on the horizontal axis.
Explanation of the saturation dome on the TV diagram, representing different phases and regions of water.
Use of the saturated water table to determine the saturation pressure at a given temperature.
Conversion of bar to kilopascals for the saturation pressure calculation.
Graphical method to determine the state of water by plotting constant pressure and temperature curves on the TV diagram.
Identification of the superheated vapor region based on pressure and temperature conditions.
Understanding that the state of water is superheated vapor when the pressure is less than the saturation pressure.
Necessity to refer to the superheated vapor table for calculations when dealing with superheated vapor.
Availability of a table for exactly 2 mega Pascals pressure and 240 degrees Celsius in the superheated vapor table.
Direct method to find the internal energy (u) from the superheated vapor table for given conditions.
Result of the internal energy calculation: 265.9 kilojoules per kilogram.
Emphasis on including units in the final result for accuracy.
Importance of practicing and mastering these calculations for analyzing systems involving water in liquid or vapor state.
Transcripts
Browse More Related Video

reading water tables
How to Use Steam Tables

How to use Steam Table - Easiest Way

Introduction to Steam Tables

Thermodynamics - Steam table example with superheated vapor, compressed liquid, liquid vapor mixture
Calorimetry Problems, Thermochemistry Practice, Specific Heat Capacity, Enthalpy Fusion, Chemistry
5.0 / 5 (0 votes)
Thanks for rating: