Thermodynamics - Steam table example with superheated vapor, compressed liquid, liquid vapor mixture
TLDRThis video script provides a detailed walkthrough on using steam tables to determine properties of water under different conditions. It covers locating states on a T-V diagram, identifying whether the state is a liquid-vapor mixture, compressed liquid, or superheated vapor, and finding specific volumes using various tables and interpolation techniques.
Takeaways
- 📚 The video aims to guide viewers through the process of using steam tables to determine various properties of water at given conditions.
- 🔍 The first example involves finding the pressure at a specific temperature (140°C) and specific volume (0.5), utilizing a temperature table to compare values.
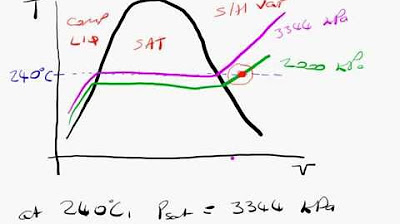
- 📈 The method starts by plotting the given conditions on a T-V (temperature-volume) diagram and identifying the location relative to the saturated water line.
- 🌡️ At 140°C, the script explains how to use the temperature table to find the corresponding pressure and specific volumes for saturated liquid (VF) and vapor (VG).
- ✂️ The specific volume given (0.5) is compared to VF and VG to determine the state of the water, which in this case is a liquid-vapor mixture.
- 📉 For the second example, the script demonstrates how to find the specific volume at a given pressure (30 MPa) and temperature (100°C), considering the difference from the saturation pressure.
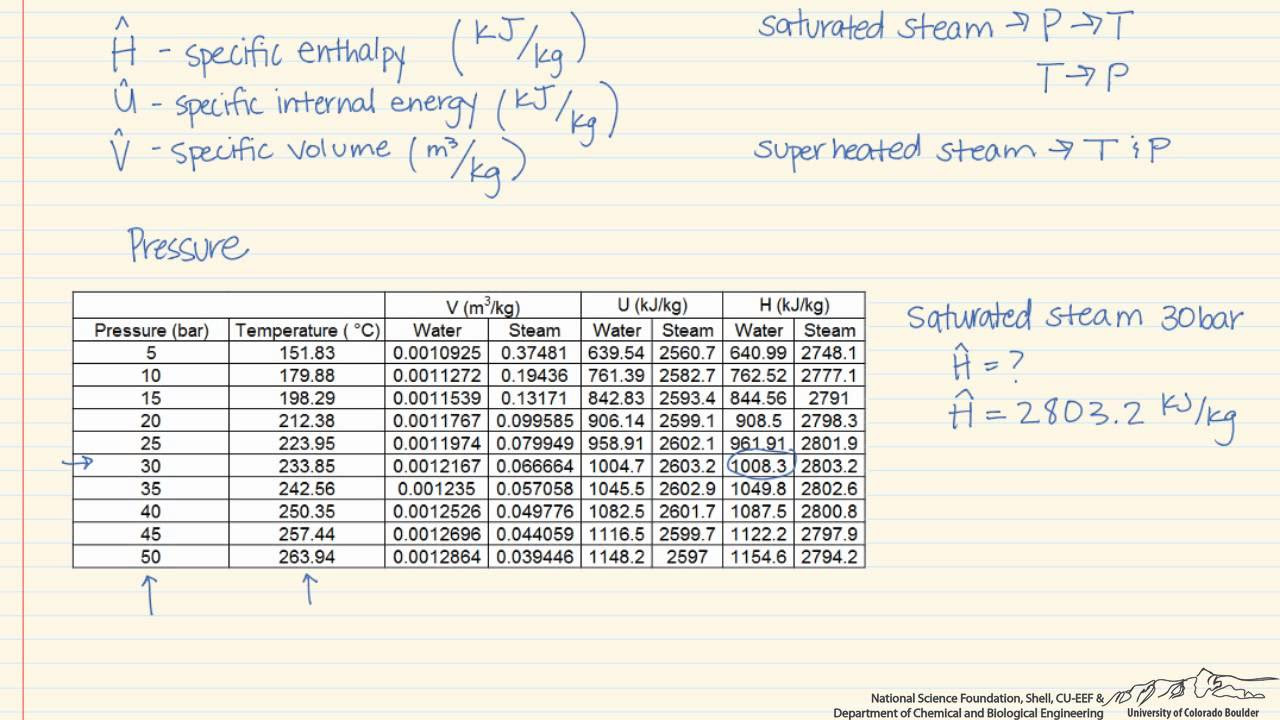
- 📊 The video explains the use of the compressed liquid table (Table A5) for conditions outside the saturation pressure, such as the 30 MPa example.
- 🔑 The importance of identifying whether the water is in a compressed liquid, liquid-vapor mixture, or superheated vapor state is emphasized for selecting the correct table.
- 🔄 The process involves interpolation when the exact temperature is not available in the table, as shown in the example with 485°C and 10 MPa.
- 📝 The script provides a formula to calculate specific volume (V) using quality (X), VF, and VG for a liquid-vapor mixture, demonstrated in the 80°C and quality 0.75 example.
- 📍 Locating the state on a T-V diagram is crucial for understanding the conditions and is illustrated in the examples provided.
Q & A
What is the purpose of the video?
-The purpose of the video is to help viewers understand how to use steam tables to find specific properties of water under various conditions and to locate the state on a T-V (temperature-volume) diagram.
What property of water is being sought in Part A of the video?
-In Part A, the property being sought is the pressure of water at a given temperature of 140 degrees Celsius and a specific volume of 0.5.
How does one determine the pressure of water when the temperature is known but the pressure is not?
-To determine the pressure when the temperature is known, one starts by locating the temperature on the saturated water table and comparing the given specific volume to the values of VF (volume of saturated liquid) and VG (volume of saturated vapor) to find the corresponding pressure.
What is the significance of the specific volume (V) in determining the state of water?
-The specific volume (V) helps determine whether the water is in a liquid, vapor, or liquid-vapor mixture state. It is compared to VF and VG to ascertain the state and find the corresponding pressure or other properties.
What is the critical pressure and temperature mentioned in the video?
-The critical pressure mentioned in the video is 220.9 bar, and the corresponding critical temperature is 374.14 degrees Celsius. These values are used to understand the limits of the liquid-vapor equilibrium state.
How is the specific volume of a compressed liquid found when given the temperature and pressure?
-The specific volume of a compressed liquid is found by referring to the compressed liquid table (Table A5 in the video) with the given temperature and pressure, and reading the specific volume value from the table.
What is the process of finding the specific volume of superheated vapor?
-To find the specific volume of superheated vapor, one must first locate the intersection of the given temperature and pressure on the T-V diagram, ensuring it is to the right of the vapor dome. Then, use the superheated vapor table (Table A4) and interpolate between the closest temperature values if necessary to find the specific volume.
What does the term 'quality' represent in the context of the video?
-In the context of the video, 'quality' represents the fraction of the total volume that is vapor in a liquid-vapor mixture. It helps determine the state of the mixture and is used to calculate the specific volume using the equation V = VF + X(VG - VF).
How can one locate the state of water on a T-V diagram given the temperature and quality?
-Given the temperature and quality, one can locate the state on a T-V diagram by first finding the corresponding pressure from the saturated water table at the given temperature, then using the quality to determine the position within the liquid-vapor mixture region, and finally marking the point on the diagram.
What is the method for finding the specific volume when the temperature and pressure are given but are not at the saturation point?
-When the temperature and pressure are given and do not correspond to the saturation point, one must determine whether the state is compressed liquid, superheated vapor, or liquid-vapor mixture by comparing the given conditions with the critical point and saturation lines on the T-V diagram. Then, use the appropriate table (A5 for compressed liquid, A4 for superheated vapor) to find the specific volume.
Outlines
🔍 Understanding Steam Tables and State Location
This paragraph introduces the concept of using steam tables to determine the properties of water under various conditions. It explains the process of locating the state of water on a T-v diagram and finding the pressure at a given temperature of 140 degrees Celsius with a specific volume of 0.5. The key steps involve consulting the saturated water tables, comparing the given specific volume to the values of VF and VG, and identifying the pressure as 3.613 MPa, which corresponds to the liquid-vapor mixture state at that temperature.
📚 Application of Steam Tables for Compressed Liquid
The second paragraph delves into finding the specific volume of water at a given pressure of 30 mega Pascals and a temperature of 100 degrees Celsius. It highlights the discrepancy between the provided pressure and the pressure required for a liquid-vapor mixture at 100 degrees Celsius according to the steam tables. The critical pressure and temperature are identified as 220.9 bar and 374.14 degrees Celsius, respectively. The paragraph guides through the process of locating the state on the T-v diagram and using the compressed liquid table (Table A5) to find the specific volume as 1.09 x 10^-3 cubic meters per kilogram.
📉 Locating Superheated Vapor State and Interpolation
This section discusses the process of identifying the state of water as superheated vapor given a pressure of 10 mega Pascals and a temperature of 485 degrees Celsius. It explains the procedure of drawing the isobar on the T-v diagram, locating the intersection with the given temperature, and using the superheated vapor table (Table A4) for finding the specific volume. Since the exact temperature is not available in the table, interpolation is used between the surrounding data points to estimate the specific volume as 0.0319 cubic meters per kilogram.
🌡️ Calculating Specific Volume with Quality and Temperature
The final paragraph focuses on calculating the specific volume of water with a given temperature of 80 degrees Celsius and a quality of 0.75. It emphasizes the importance of understanding the quality parameter in determining the state of water as a liquid-vapor mixture. Using the saturated water table, the specific volumes for saturated liquid (VF) and vapor (VG) are identified. The paragraph demonstrates the calculation of specific volume using the formula V = VF + X(VG - VF), resulting in a specific volume of 2.556 cubic meters per kilogram. It also describes how to locate this state on the T-v diagram.
Mindmap
Keywords
💡Steam Tables
💡Thermodynamic Properties
💡P-v Diagram
💡Saturated Water Table
💡Specific Volume
💡Quality
💡Mega Pascal
💡
💡Compressed Liquid
💡Superheated Vapor
💡Interpolation
💡Critical Pressure
Highlights
Introduction to using steam tables for understanding thermodynamic properties of water at various conditions.
Explanation of how to choose the appropriate steam table based on given conditions.
Demonstration of locating the state of water on a T-v diagram for a given temperature and specific volume.
Method to find pressure when only temperature and specific volume are known.
Utilization of the saturated water table to compare values and determine pressure at 140 degrees Celsius.
Conversion of specific volume units from m³/kg to more practical units.
Identification of the state as a liquid-vapor mixture by comparing specific volume to VF and VG.
Explanation of how to determine the pressure for a liquid-vapor mixture at a given temperature.
Transition to using a different table when specific volume is outside the range of VF and VG.
Process of finding specific volume given pressure and temperature for a compressed liquid.
Use of the compressed liquid table (table A5) for calculations when pressure is higher than the saturation pressure.
Interpolation method for estimating specific volume when exact temperature is not available in the table.
Identification of the state as superheated vapor by its location on the T-v diagram.
Application of the superheated vapor table (table A4) for finding specific volume at high temperatures and low pressures.
Explanation of how to use quality to find specific volume in a liquid-vapor mixture.
Calculation of specific volume using the formula V = VF + X(VG - VF) with given quality.
Visual representation of the state location on the T-v diagram for a liquid-vapor mixture with quality.
Conclusion summarizing the process of using steam tables to identify the state of water and calculate specific volume.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: