reading water tables
TLDRThis screencast explains how to use water tables to determine properties of water at different temperatures and pressures. It covers saturated water tables organized by temperature and pressure, and superheated water tables. The script demonstrates how to identify states like saturated liquid, vapor, and superheated vapor using specific volume, internal energy, enthalpy, and entropy.
Takeaways
- 🌡️ The script discusses how to use water tables, specifically for selecting the appropriate table to solve problems related to water properties.
- 🔍 There are two primary types of water tables: one organized by temperature (saturated water temperature table) and another by pressure (saturated water pressure table).
- 📊 Both saturated temperature and pressure tables contain the same information but are organized differently for ease of use.
- 🌡️ At a given pressure, the saturation temperature can be found in the saturated water pressure table, and vice versa.
- 🔥 The script explains how to identify the state of water (e.g., superheated vapor) based on temperature and pressure values.
- 🌡️ For a fluid at 200 kilopascals and 300°C, the state is identified as superheated vapor, requiring reference to superheated water tables.
- 📚 Superheated water tables are organized by pressure and temperature, providing properties like specific volume, internal energy, enthalpy, and entropy.
- 💧 The saturation temperature at a given pressure is indicated in parentheses in the superheated water tables, showing the relationship between pressure and saturation.
- 🌡️ The script provides an example of finding water properties at 100°C and 50 kilopascals, identifying it as superheated vapor due to the pressure being lower than the saturation pressure.
- 🔍 The internal energy of a system can be used to determine its state (e.g., superheated vapor) by comparing it with values in the saturated water pressure table.
- 🌡️ An example problem is solved where the temperature is determined to be about 973°C for water at 100 kilopascals and an internal energy of 4,000 K per kilogram.
Q & A
What is the purpose of the saturated water temperature table?
-The saturated water temperature table organizes data by specific temperatures, allowing users to find properties such as specific volume, internal energy, enthalpy, and entropy at a given temperature.
How is the saturated water table organized by pressure different from the one organized by temperature?
-The saturated water table organized by pressure has pressure in kilopascals in the first column, while the table organized by temperature has temperature in the first column. Both tables contain the same information but are organized differently for convenience.
What does the line at 20 kilopascal on the saturated water pressure table represent?
-The line at 20 kilopascal on the saturated water pressure table represents the properties of water at that pressure, including its saturation temperature of about 60°C.
What is the significance of the notation 'f' and 'g' in the tables?
-The notation 'f' stands for saturated liquid, and 'g' stands for saturated vapor. These notations help differentiate between the properties of saturated liquid and saturated vapor.
Why is the evaporation column in the tables considered redundant?
-The evaporation column is redundant because it represents the difference between the internal energy of the vapor and the internal energy of the fluid, which can be calculated directly from the values provided for saturated liquid and vapor.
What state of water is described at 200 kilopascal and 300°C?
-At 200 kilopascal and 300°C, the water is in a superheated vapor state, as the temperature is greater than the saturation temperature of 12.21°C at that pressure.
How can you determine the properties of superheated water?
-The properties of superheated water can be determined by using the superheated water tables, which provide specific volume, internal energy, enthalpy, and entropy at various temperatures and pressures.
What do the temperatures in parentheses in the superheated water tables represent?
-The temperatures in parentheses in the superheated water tables represent the saturation temperature at the given pressure.
Why is the pressure of 50 kilopascal at 100°C considered a superheated vapor?
-At 100°C, the saturation pressure is 10.42 kilopascal. A pressure of 50 kilopascal is lower than this, indicating that the vapor is superheated as it does not have enough pressure to condense into a liquid.
How can you find the temperature of a system with a pressure of 100 kilopascal and an internal energy of 4,000 K per kilogram?
-By consulting the superheated vapor tables at 100 kilopascal, you can find the temperature by locating the internal energy value of 4,000 K per kilogram and interpolating between the closest temperatures provided.
Outlines
📊 Understanding Water Tables for Thermodynamic Properties
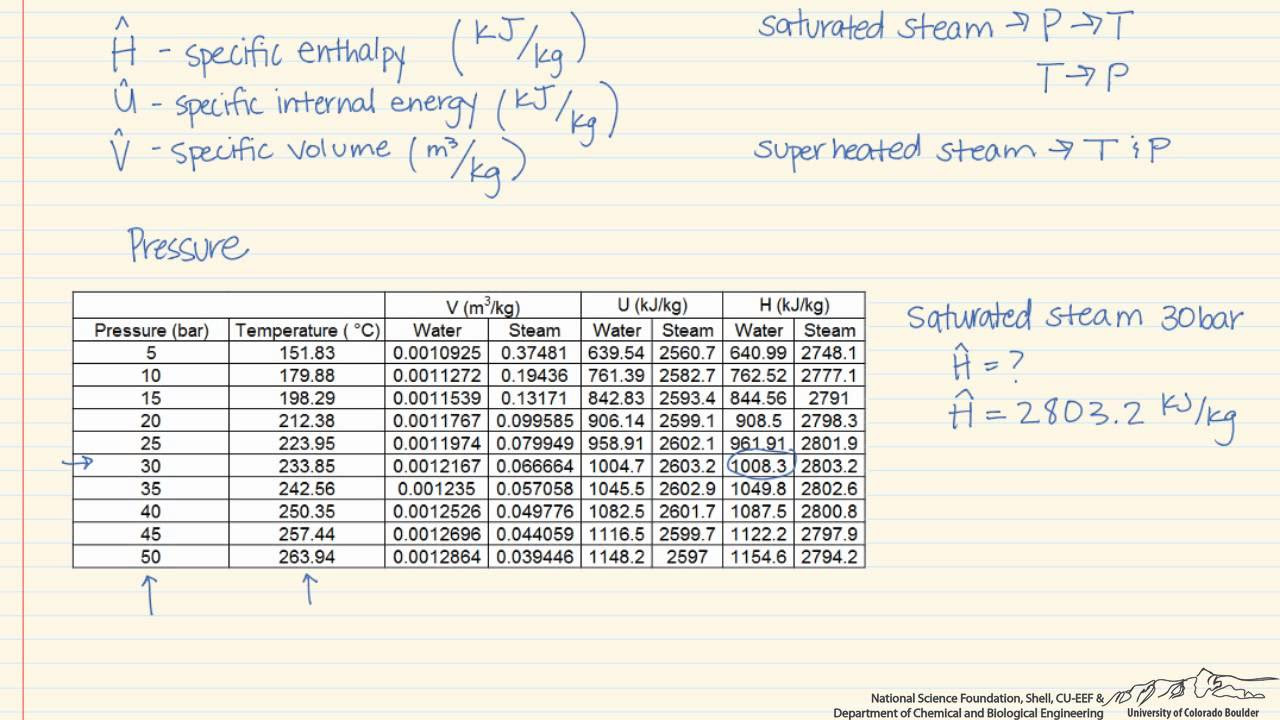
This paragraph introduces the concept of water tables used for determining thermodynamic properties of water at various temperatures and pressures. It explains the two primary types of saturated water tables: one organized by temperature and the other by pressure. The paragraph details how to use these tables to find properties like specific volume, internal energy, enthalpy, and entropy. It also clarifies that while the tables contain the same information, they are organized differently for convenience. The speaker uses examples to illustrate how to find the saturation temperature and pressure, and how to interpret data for both saturated liquid and vapor states.
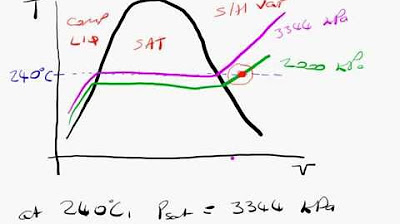
🔍 Selecting Appropriate Water Tables for Superheated Vapor
The second paragraph focuses on how to identify and use superheated water tables to determine the properties of water when it is in a superheated vapor state. It explains the process of locating the correct table based on given pressure and temperature conditions that exceed the saturation point. The paragraph provides an example of finding specific volume, internal energy, enthalpy, and entropy for superheated water at 200 kilopascals and 300°C. It also discusses the significance of temperatures in parentheses, which represent the saturation temperature at a given pressure, and how these can be used for comparison with superheated states.
🔬 Determining Water State and Properties Using Given Data
The final paragraph demonstrates how to use provided data, such as pressure and temperature or internal energy, to ascertain the state and properties of water. It walks through the process of identifying whether the water is a superheated vapor based on its internal energy exceeding that of saturated vapor. The paragraph includes an example of finding the temperature of a system with a given internal energy of 4,000 K per kilogram at a pressure of 100 kilopascals. It concludes with an interpolation method to estimate the temperature based on the internal energy values found in the superheated vapor tables.
Mindmap
Keywords
💡Water Tables
💡Saturated Water
💡Superheated Vapor
💡Specific Volume
💡Internal Energy (U)
💡Enthalpy (H)
💡Entropy (S)
💡Quality
💡Subscripts f and g
💡MegaPascal (MPa)
💡Interpolation
Highlights
Introduction to using water tables for solving problems.
Explanation of the saturated water temperature table and its organization by temperature.
Description of how to find properties like specific volume, internal energy, enthalpy, and entropy using the saturated water temperature table.
Introduction to the saturated water table organized by pressure.
Comparison of the two saturated tables and their organization differences.
Demonstration of how to read data from the saturated water pressure table.
Explanation of how to correlate pressure and temperature in the saturated water tables.
Discussion on the notation used for saturated liquid and vapor in the tables.
Clarification of the redundant nature of the evaporation column in the tables.
Example problem identifying the state of water at 200 kilopascal and 300° C.
Use of superheated water tables for properties of water in non-saturated conditions.
Identification of superheated vapor state in water at high temperatures.
Explanation of how to find properties of superheated water using temperature and pressure.
Note on the significance of saturation temperature in parenthesis in superheated water tables.
Problem-solving example with water at 100° C and 50 kilopascal.
Discussion on the concept of superheated vapor in relation to pressure and temperature.
Example problem using specific internal energy to determine the temperature of a system.
Use of superheated vapor tables to find temperature based on internal energy and pressure.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: