3.1 Introduction to Acids and Bases | Organic Chemistry
TLDRThis lesson delves into the world of acids and bases from an organic chemistry perspective, offering a comprehensive understanding of their definitions and roles in chemical reactivity. The video begins with a review of the Arrhenius definition and then emphasizes the importance of the Brønsted-Lowry and Lewis definitions for understanding organic chemistry. The Brønsted-Lowry definition focuses on the transfer of protons (H+), identifying acids as proton donors and bases as proton acceptors. In contrast, the Lewis definition shifts the focus to electron pairs, with acids being electron acceptors and bases as electron donors. The lesson also explores the concept of conjugate acids and bases, highlighting the inverse relationship between their strengths and the stability of the conjugate base. Strengths of acids and bases are typically measured using the equilibrium constant (Ka) and its negative logarithm (pKa), with lower pKa values indicating stronger acids. The video provides a list of pKa values for various functional groups, emphasizing the need to memorize these for accurate comparison and ranking of acids and bases. Lastly, the lesson discusses how to predict the position of equilibrium in acid-base reactions, noting that weaker acids and bases are favored at equilibrium. The presenter encourages viewers to subscribe for weekly lessons throughout the 2020-21 school year and to utilize study guides and practice problems for further understanding.
Takeaways
- 📚 Acids and bases are central to understanding chemical reactivity in organic chemistry, building upon general chemistry concepts.
- 🔍 The Bronsted-Lowry definition views acids as hydrogen ion (H+) donors and bases as H+ acceptors, focusing on the transfer of H+ ions.
- 🔬 The Lewis definition considers acids as electron pair acceptors and bases as electron pair donors, emphasizing the movement of electrons rather than just H+ ions.
- 🔢 The strength of an acid is often measured by its Ka value, with a higher Ka indicating a stronger acid. In organic chemistry, the pKa (negative log of Ka) is more commonly used, where a lower pKa indicates a stronger acid.
- ⚖️ The strength of an acid is inversely related to the strength of its conjugate base; a stronger acid has a weaker conjugate base.
- 🧠 Memorizing pKa values for various functional groups is crucial for comparing the strengths of acids and bases in organic chemistry.
- 📉 A larger difference in pKa values between two acids or bases indicates a greater favoring of the weaker acid and base at equilibrium.
- 🔄 At equilibrium, acid-base reactions favor the formation of the weaker acid and base, which are more stable.
- 🚦 The relationship between an acid and its conjugate base is important for understanding the equilibrium of acid-base reactions.
- 📝 Students are encouraged to subscribe to the channel and utilize study guides and practice problems for a deeper understanding of acids and bases.
- 🌟 For additional resources, including a premium course, students can visit chatsprep.com for more practice on acids and bases.
Q & A
What is the main topic of the lesson?
-The main topic of the lesson is acids and bases from an organic chemistry perspective.
What are the two definitions of acids and bases that are pivotal in understanding organic chemistry?
-The two pivotal definitions are the Brønsted-Lowry definition, which focuses on hydrogen ion (H+) transfer, and the Lewis definition, which emphasizes electron pair transfer.
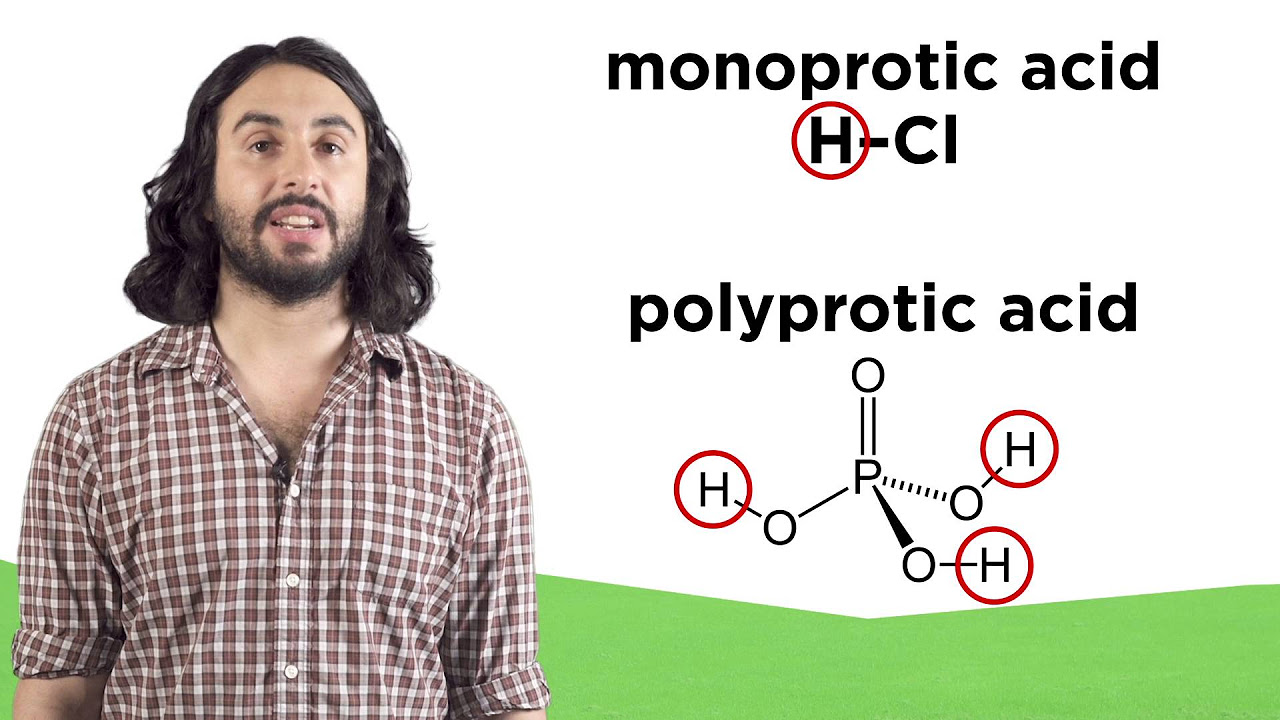
According to the Brønsted-Lowry definition, what is considered an acid and what is considered a base?
-In the Brønsted-Lowry definition, an acid is considered to be an H+ donor and a base is considered to be an H+ acceptor.
How does the Lewis definition differ from the Brønsted-Lowry definition in terms of acid-base reactions?
-The Lewis definition differs by focusing on electron pair acceptance and donation, rather than just the transfer of H+ ions.
What is the relationship between the strength of an acid and its conjugate base?
-The stronger the acid, the weaker its conjugate base, and vice versa. This inverse relationship is due to the stability of the species involved.
Why is it important to know the pKa values of different functional groups in organic chemistry?
-The pKa values are important because they help in ranking the relative strengths of acids and are commonly used in organic chemistry to compare different acids.
What does a lower pKa value indicate in terms of acid strength?
-A lower pKa value indicates a stronger acid because it means the acid dissociates more in water, leading to a higher concentration of H+ ions.
How does the equilibrium of an acid-base reaction relate to the strength of the acids and bases involved?
-At equilibrium, the weaker acid and base are favored, meaning that the reaction will tend to have more reactants present if the stronger acid and base are on the product side.
What is the role of the stability of the conjugate base in ranking acids?
-The stability of the conjugate base is often used to rank acids. The more stable the conjugate base, the weaker the acid, and vice versa.
Why is it recommended to memorize the pKa values of various functional groups before applying ranking rules?
-Memorizing the pKa values helps to quickly identify the relative strengths of acids and bases without relying on rules that may have exceptions. It provides a solid foundation for understanding acid-base reactivity.
How does the instructor suggest determining the favored side of an acid-base equilibrium?
-The instructor suggests comparing the pKa values of the acids or bases involved. At equilibrium, the side with the weaker acid and base is favored.
Outlines
🔬 Introduction to Acids and Bases in Organic Chemistry
This paragraph introduces the topic of acids and bases from an organic chemistry perspective. It emphasizes the importance of understanding the molecular characteristics that allow for the ranking of acids and bases. The lesson is part of a new organic chemistry playlist, with weekly releases during the 2020-21 school year. The speaker reminds viewers of the Arrhenius definition of acids and bases and highlights the pivotal role of the Bronsted-Lowry and Lewis definitions in organic chemistry. The Bronsted-Lowry definition focuses on hydrogen ion (H+) transfer, identifying acids as H+ donors and bases as H+ acceptors. The Lewis definition, which is particularly important for organic chemistry, views acids as electron acceptors and bases as electron donors, focusing on the formation of new bonds and the movement of electrons. The paragraph also explains that a reaction can be classified as both a Bronsted-Lowry and a Lewis acid-base reaction if the base bonds to a hydrogen atom, but if it bonds to another atom, like carbon, it would only be a Lewis acid-base reaction.
📊 Strength of Acid-Base Reactions and the Role of pKa
This paragraph discusses how the strength of acid-base reactions is typically measured by their degree of dissociation in water. It introduces the equilibrium constant, Ka, and explains that a larger Ka value indicates a stronger acid. In organic chemistry, the negative logarithm of Ka, known as pKa, is more commonly used, where a lower pKa signifies a stronger acid. The relationship between an acid and its conjugate base is also explored, noting that the strength of an acid inversely correlates with the strength of its conjugate base. The paragraph further explains that stronger acids have weaker conjugate bases and more stable conjugate bases, which is crucial for ranking acids. It concludes with a list of various functional groups and their corresponding pKa values, emphasizing the need to memorize these values for ease in ranking acids and bases.
🔍 Recognizing Structural Elements and pKa Values for Acid-Base Comparisons
This paragraph focuses on the importance of memorizing pKa values for various functional groups to compare and rank acids and bases effectively. It provides a list of functional groups, ranging from sulfonic acids with a very low pKa to ammonia with a high pKa, indicating their relative acid strengths. The paragraph also discusses the concept of equilibrium in acid-base reactions, explaining that the weaker acid and base are favored at equilibrium. It illustrates how to determine the favored side of the equilibrium by comparing the pKa values of the acids involved in the reaction. The speaker advises using memorized pKa values first and then applying ranking rules, as there will always be exceptions to any set of rules. The paragraph concludes with an encouragement to like, share, and support the channel, and to check out the premium course for further practice on acid-base chemistry.
Mindmap
Keywords
💡Acids and Bases
💡Brønsted-Lowry Definition
💡Lewis Definition
💡pKa
💡Conjugate Acid-Base Pair
💡Equilibrium Constant (Ka)
💡Organic Chemistry
💡Sulfonic Acid
💡Carboxylic Acid
💡Phenol
💡Alcohol
Highlights
Lesson introduces acids and bases from an organic chemistry perspective, building on general chemistry knowledge.
Emphasis on the importance of understanding Bronsted-Lowry and Lewis definitions for grasping organic chemistry concepts.
Explanation of how acids are H+ donors and bases are H+ acceptors in Bronsted-Lowry theory.
Introduction to Lewis definition, where acids are electron acceptors and bases are electron donors.
Discussion on the significance of following electrons to understand bond formation in Lewis acid-base reactions.
Crucial role of understanding acid-base reactions for grasping chemical reactivity in organic chemistry.
Use of pKa values, rather than Ka, for ranking acid strengths in organic chemistry.
Inverse relationship between the strength of an acid and its conjugate base, with stability implications.
Memorization of pKa values for various functional groups is key for comparing acid strengths without relying solely on rules.
Practical approach to identifying stronger acids and bases by comparing pKa values of functional groups involved in reactions.
Illustration of how the difference in pKa values influences the favoring of products or reactants in acid-base equilibrium.
Application of Bronsted-Lowry's definition to predict the favoring of reactants or products in typical acid-base reactions.
The principle that weaker acids and bases are favored at equilibrium in acid-base reactions.
Utility of comparing conjugate bases for acid ranking due to the inverse strength relationship.
Importance of recognizing exceptions to acid-base ranking rules and the value of memorizing pKa values.
Introduction of various functional groups and their pKa values for organic chemistry students to memorize.
Guidance on using pKa values and structural rules to rank acids and bases effectively in organic chemistry.
Advice for using the study guide and premium course for practice problems on acids and bases.
Transcripts
Browse More Related Video

Conjugate Acid Base Pairs, Arrhenius, Bronsted Lowry and Lewis Definition - Chemistry

Introduction to Acid-Base Chemistry - AP Chemistry Unit 4, Topic 8

Acids and Bases - Basic Introduction - Organic Chemistry
Acids and Bases, pH and pOH

16.1 Introduction to Acids and Bases | General Chemistry

Acidity: Crash Course Organic Chemistry #11
5.0 / 5 (0 votes)
Thanks for rating: