Introduction to Acid-Base Chemistry - AP Chemistry Unit 4, Topic 8
TLDRIn this educational video, Jeremy Krug delves into the fundamentals of acids and bases in AP Chemistry. He explains the traditional definitions, highlighting their properties like sour taste for acids and slippery feel for bases, and introduces the concept of Arrhenius acids and bases, which dissociate into hydrogen and hydroxide ions respectively. Krug then transitions to the more inclusive Bronsted-Lowry definition, describing acids as proton donors and bases as proton acceptors, illustrating this with examples of acid-base reactions and the formation of conjugate acid-base pairs. The video offers a foundational understanding of acid-base chemistry, setting the stage for deeper exploration in Unit 8.
Takeaways
- 🍋 Acids are known for their corrosive properties and can be found in foods like vinegar and citric acid, giving them a sour taste.
- 🧼 Bases are recognized for their cleansing properties and are commonly found in soaps, drain cleaners, and window cleaners, often having a slippery feel.
- 🔬 A Swedish chemist named Sante Arrhenius is credited with the initial definitions of acids as substances that produce hydrogen ions (H+) and bases as those that produce hydroxide ions (OH-).
- 📚 Arrhenius acids and bases dissociate into their component ions when added to water, such as HCl breaking into H+ and Cl- ions.
- 🧪 An example problem demonstrates how to calculate the concentration of hydroxide ions in a solution of calcium hydroxide, using stoichiometry.
- 🚫 The Arrhenius definition is limiting as it excludes many substances that are acids or bases in the real world, such as weak acids and bases.
- 🌐 The Bronsted-Lowry definition is more inclusive, defining acids as proton donors (H+) and bases as proton acceptors, broadening the scope of what can be considered an acid or a base.
- 🔄 In acid-base reactions, there are always two conjugate acid-base pairs, differing by one proton, which can be identified in any given reaction.
- 💧 Water is an amphoteric substance, meaning it can act as both an acid and a base, depending on the reaction it is involved in.
- 🔬 The script introduces the concept of conjugate acid-base pairs, which are closely related and differ by one H+ ion.
- 📚 The video is part of a series on AP Chemistry, with a deeper dive into acid-base chemistry planned for Unit 8.
Q & A
What is the main topic of the video by Jeremy Krug?
-The main topic of the video is to deepen the understanding of acids and bases in the context of AP Chemistry.
What are some common properties of acids mentioned in the video?
-Acids are known for corroding metals, being used for cleaning, and having a sour taste when found in dilute forms like vinegar and citric acid in foods.
What is a common example of a base that people encounter daily?
-Soap is a common example of a base that people encounter daily due to its cleansing properties.
How does the video describe the chemical definition of an acid?
-The video describes an acid chemically as anything that produces hydrogen ions (H+) in a solution.
What is the general formula characteristic of an acid according to the video?
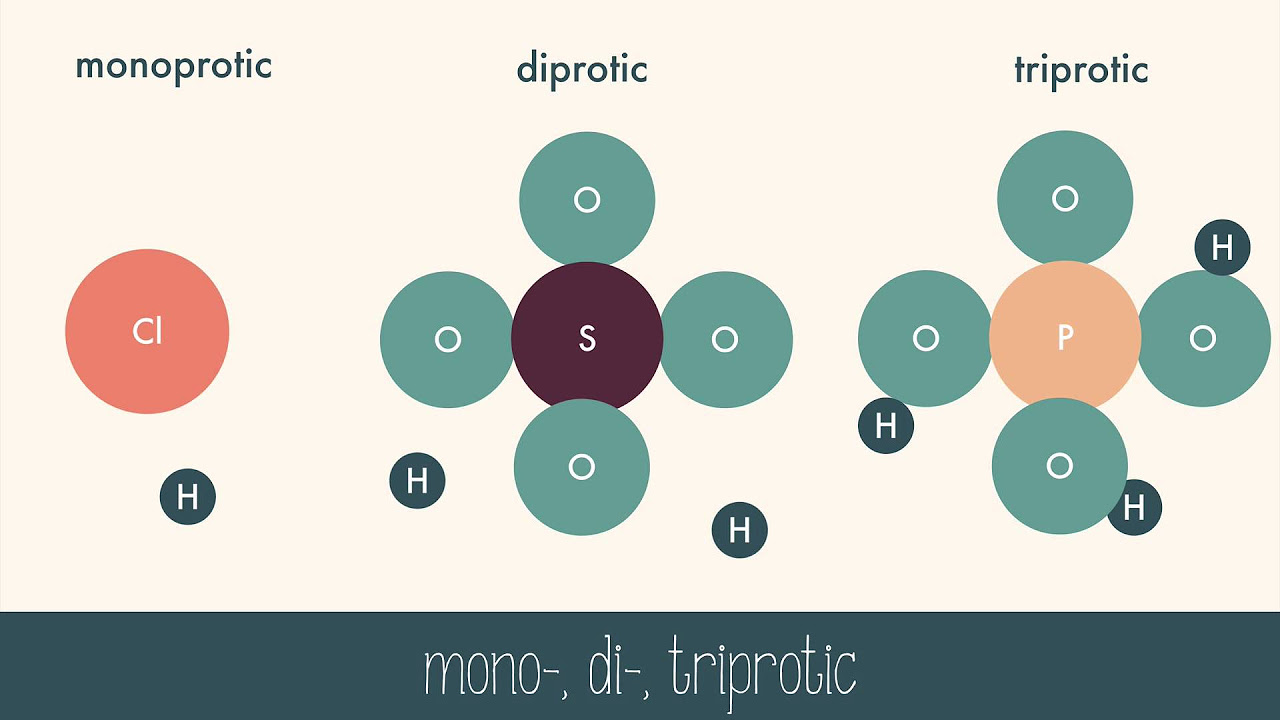
-The general formula characteristic of an acid is that it starts with hydrogen, such as in Hydrochloric (HCl), hydrofluoric (HF), and nitric acid (HNO3).
What is the chemical definition of a base as explained in the video?
-A base is defined chemically as anything that produces hydroxide ions (OH-) in a solution.
What is the significance of the ratio in the dissociation of calcium hydroxide mentioned in the video?
-The significance of the ratio is that for every mole of calcium hydroxide, there are two moles of hydroxide ions produced due to the 1:2 ratio in its dissociation.
Who is credited with the initial descriptions of acids and bases as mentioned in the video?
-Swedish chemist Sante Arrhenius is credited with the initial descriptions of acids and bases.
What is the broader definition of acids and bases that the video mentions for AP Chemistry?
-The broader definition mentioned in the video is the one produced by Johannes Brønsted and Thomas Lowry, where an acid is a proton donor and a base is a proton acceptor.
What is the term used for substances that can act as both an acid and a base?
-Substances that can act as both an acid and a base are called amphoteric.
What is the role of water in acid-base reactions as depicted in the video?
-Water can act as either an acid or a base in different reactions, depending on whether it is donating or accepting protons (H+).
How does the video explain the concept of conjugate acid-base pairs?
-The video explains that in every acid-base reaction, there are two conjugate acid-base pairs, where each acid in the pair has one more H+ than its corresponding base.
Outlines
🧪 Basics of Acids and Bases in Chemistry
Jeremy Krug introduces the concept of acids and bases in AP Chemistry, explaining their common properties and uses. Acids are known for their corrosive nature and sour taste, while bases are recognized for their cleansing properties and slippery feel. The video discusses the chemical definitions of acids as substances that produce hydrogen ions (H+) and bases as those that produce hydroxide ions (OH-). It also touches on the historical contributions of Sante Arrhenius in defining acids and bases and provides an example of how to calculate hydroxide ion concentration in a solution.
🔍 Expanding the Definition of Acids and Bases
The script moves on to discuss the limitations of the Arrhenius definition and introduces the more inclusive Bronsted-Lowry definition of acids and bases. According to this definition, an acid is a proton (H+) donor, and a base is a proton acceptor. The video illustrates how this definition applies to various reactions, including the formation of hydronium ions (H3O+) from water, and the concept of conjugate acid-base pairs, which differ by a single proton. The script also provides an example of identifying acids and bases in a given chemical reaction.
🌐 Further Exploration of Acid-Base Reactions
In this section, the script delves deeper into acid-base reactions, using the dissociation of ammonia in water as an example. It explains how to write the base dissociation equation and identify the roles of water as both an acid and a base in different contexts, highlighting the concept of amphoteric substances. The video also discusses the identification of conjugate acid-base pairs in reactions and emphasizes the importance of understanding these concepts for further study in Unit 8 of AP Chemistry.
Mindmap
Keywords
💡Acids
💡Bases
💡Arrhenius Acids and Bases
💡Hydrogen Ion
💡Hydroxide Ion
💡Dissociation
💡Conjugate Acid-Base Pairs
💡Bronsted-Lowry Definition
💡Proton Donor and Acceptor
💡Amphoteric Substances
💡Dissociation Equation
Highlights
Introduction to deepening understanding of acids and bases in AP Chemistry.
Basic properties of acids: corroding metals, cleaning, and sour taste.
Basic properties of bases: cleansing properties, slippery feel, and common household uses.
Chemical definition of an acid: produces hydrogen ions in a solution.
Recognition of acids by their chemical formula starting with hydrogen.
Chemical definition of a base: produces hydroxide ions in a solution.
Recognition of bases by their chemical formula ending with hydroxide.
Historical context: Sante Arrhenius' contribution to the definition of acids and bases over 100 years ago.
Dissociation of Arrhenius acids and bases in water into their component ions.
Example problem: Calculating hydroxide ion concentration in a calcium hydroxide solution.
Limitations of the Arrhenius definition and the need for a more inclusive definition.
Introduction of the Brønsted-Lowry definition of acids and bases as proton donor and acceptor.
Explanation of the concept of conjugate acid-base pairs differing by one proton.
Example of identifying acids, bases, and their conjugates in chemical reactions.
Demonstration of water acting as both an acid and a base, highlighting its amphoteric nature.
Dissociation of ammonia in water as an example of a base accepting a proton.
Identification of conjugate acid-base pairs in the dissociation of ammonia.
Upcoming coverage of acid-base chemistry in Unit 8 with a focus on Brønsted-Lowry definitions.
Transcripts
Browse More Related Video

3.1 Introduction to Acids and Bases | Organic Chemistry

Conjugate Acid Base Pairs, Arrhenius, Bronsted Lowry and Lewis Definition - Chemistry

Arrhenius definition of acids and bases | Biology | Khan Academy
Acid-Base Theories

AP Chem - Unit 8 Review - Acids and Bases in 10 Minutes - 2023

Acid-Base Reactions and pH Calculations - AP Chem Unit 8, Topic 4
5.0 / 5 (0 votes)
Thanks for rating: