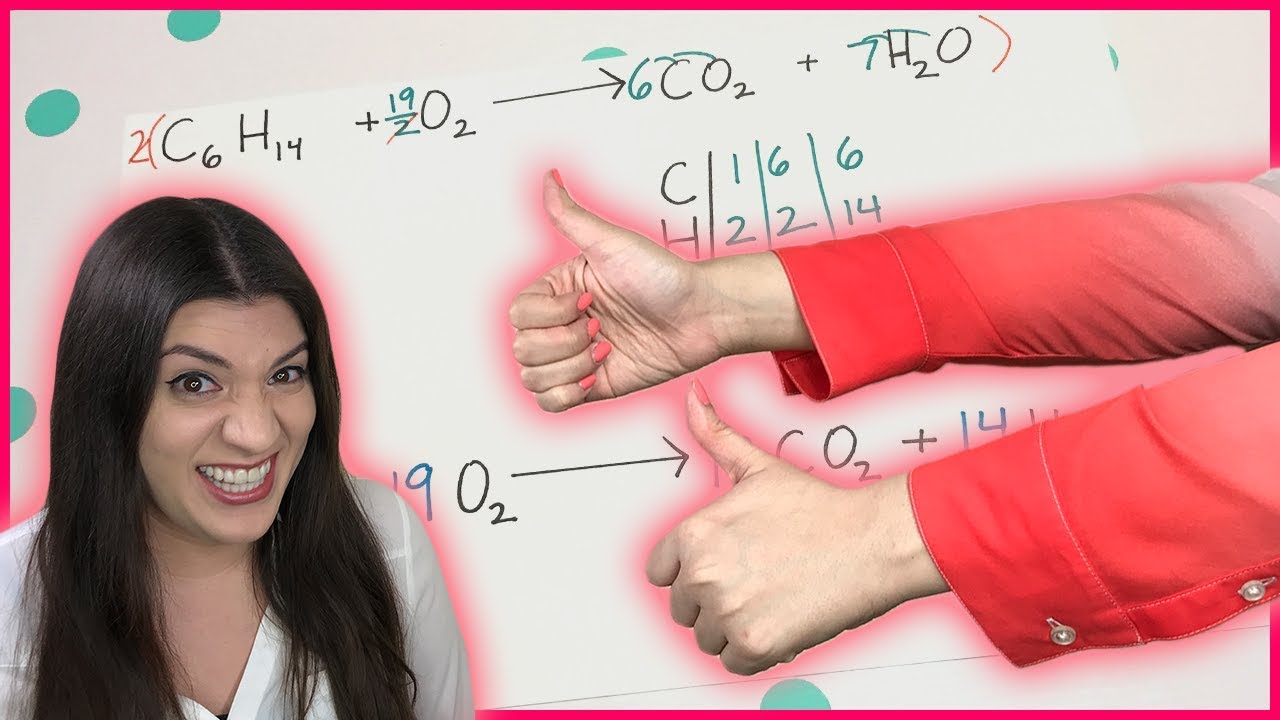
How to Balance Chemical Equations
TLDRThis video script offers a step-by-step guide on balancing chemical equations. It emphasizes counting atoms, starting with metals, then nonmetals, and finally hydrogen or other elements. The script illustrates the process with three examples, showing how to apply coefficients to achieve balance. It concludes with a reminder to spread kindness, aligning with the educational content.
Takeaways
- 📚 Start by drawing a line down the middle to separate reactants and products in a chemical equation.
- 🔍 Count the atoms of each element on both sides of the equation to ensure they are equal.
- 🧑🔬 Begin balancing with metals, then nonmetals, and lastly hydrogen or any other elements.
- ⚖️ Apply coefficients to the chemical formulas to balance the number of atoms for each element.
- 🔢 Use the smallest common multiple to determine the necessary coefficients for balancing oxygen atoms.
- 🛠️ For the first equation, a coefficient of 2 was added to hydrogen to balance the equation.
- 🌟 In the second equation, a coefficient of 3 was used for iron and 2 for the oxygen on the reactant side to balance the equation.
- 📉 For the third equation, a coefficient of 2 was initially used for potassium, and then adjustments were made to chlorine and oxygen to achieve balance.
- 🧠 Remember that subscripts only apply to the element they are attached to, not to the entire formula.
- 🔄 After balancing one element, check and adjust other elements as needed to maintain balance.
- ❤️ The video concludes with a reminder to be kind, emphasizing that kindness multiplies.
Q & A
What is the first step recommended in the script for balancing chemical equations?
-The first step recommended is to draw a line down the middle and then count the atoms on both sides of the equation.
How does the script suggest to approach the balancing of a chemical equation?
-The script suggests starting with the metals, then nonmetals, and finally hydrogen or any other elements present.
What does the script imply by 'remember a capital letter means you have a new element'?
-It implies that in chemical notation, an element represented by a capital letter indicates the start of a new element, distinct from others.
How does the script handle the balancing of hydrogen in the first chemical equation example?
-The script adds a coefficient of 2 to the hydrogen on the side with only one hydrogen atom to balance the hydrogen atoms on both sides.
What is the strategy used in the script to balance chlorine atoms in the first equation?
-The script multiplies the chlorine atom by 2 on the side with only one chlorine atom, resulting in two chlorine atoms which balances with the two chlorine atoms on the other side.
What does the script suggest as the starting point for balancing the second chemical equation?
-The script suggests starting with the metal, which in this case is iron, and then balancing the other elements accordingly.
How does the script determine the coefficient for oxygen in the second chemical equation?
-The script finds the smallest number that both oxygen quantities can be divided by, which is 4, and applies this as a coefficient to balance the oxygen atoms.
What is the initial step taken in the script to balance the third chemical equation?
-The script starts by multiplying the potassium by 2 to match the two potassium atoms on the other side of the equation.
How does the script resolve the imbalance of oxygen atoms in the third chemical equation?
-The script changes the coefficient of oxygen from 3 to 4, which is a multiple of 12, to balance the 6 oxygen atoms on the other side.
What is the final advice given in the script that is unrelated to chemistry?
-The final advice given is to be kind to someone, emphasizing that kindness multiplies.
Outlines
🔬 Introduction to Balancing Chemical Equations
In this video, we will learn how to balance chemical equations. The process begins with drawing a line down the middle of the equation and counting the atoms on each side. For the first example, we have zinc, hydrogen, and chlorine atoms on both sides of the equation. By adding a coefficient to balance the hydrogen and chlorine atoms, we achieve a balanced equation. The key steps include identifying the elements, counting atoms, and using coefficients to balance the equation.
🧪 Example of Balancing Iron and Oxygen
The second example involves balancing an equation with iron and oxygen. Starting by counting the atoms of each element on both sides, we find that adding a coefficient to iron balances the metals. Then, we balance the oxygen by finding the smallest common multiple, ensuring both sides have the same number of oxygen atoms. The balanced equation demonstrates the method of systematically adjusting coefficients to achieve balance.
🔍 Balancing Potassium, Chlorine, and Oxygen
In the final example, we balance an equation with potassium, chlorine, and oxygen. By drawing a line and counting atoms, we identify the need to adjust coefficients for potassium and chlorine first. The challenge is to balance the oxygen atoms by finding a common multiple. After multiple adjustments, the equation is balanced with careful consideration of each element's count. This example highlights the iterative process of balancing complex equations.
📊 Summary and Key Tips for Balancing Equations
To summarize, the process of balancing chemical equations involves drawing a line to separate the two sides, counting the atoms, and starting with metals, followed by nonmetals and other elements like hydrogen. The video concludes with a reminder to be kind and a motivational message. The structured approach and examples provided aim to make balancing equations clear and manageable.
Mindmap
Keywords
💡Balance Equations
💡Atoms
💡Capital Letter
💡Subscript
💡Metals
💡Nonmetals
💡Coefficient
💡Hydrogen
💡Chlorine
💡Oxygen
💡Kindness
Highlights
Introduction to balancing chemical equations by drawing a line down the middle and counting atoms.
Starting with the metal elements in a chemical equation before moving to nonmetals and then hydrogen.
Balancing the zinc and hydrogen atoms in the first example equation by applying a coefficient.
Ensuring chlorine atoms are balanced after adjusting the hydrogen coefficient.
Counting atoms of iron and oxygen in the second example equation.
Balancing the metal atoms by multiplying the iron coefficient in the second equation.
Using the smallest common multiple to balance oxygen atoms in the second equation.
Counting potassium, chlorine, and oxygen atoms in the third example equation.
Balancing potassium atoms by adjusting the coefficient to match the other side of the equation.
Addressing the chlorine imbalance by ensuring both sides of the equation have an equal number of chlorine atoms.
Balancing oxygen atoms by finding the least common multiple and adjusting coefficients accordingly.
Final adjustment of coefficients to ensure all elements are balanced in the third equation.
Summary of the process: drawing a line, counting atoms, starting with metals, then nonmetals, and finally hydrogen or other elements.
Emphasizing the importance of kindness and its multiplying effect as a concluding message.
Encouraging viewers to be kind to someone today as a parting thought.
Musical elements used to enhance the educational content of the video.
Visual demonstration of the step-by-step process of balancing chemical equations.
Explanation of the significance of subscripts and how they apply only to the specific element they follow.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: