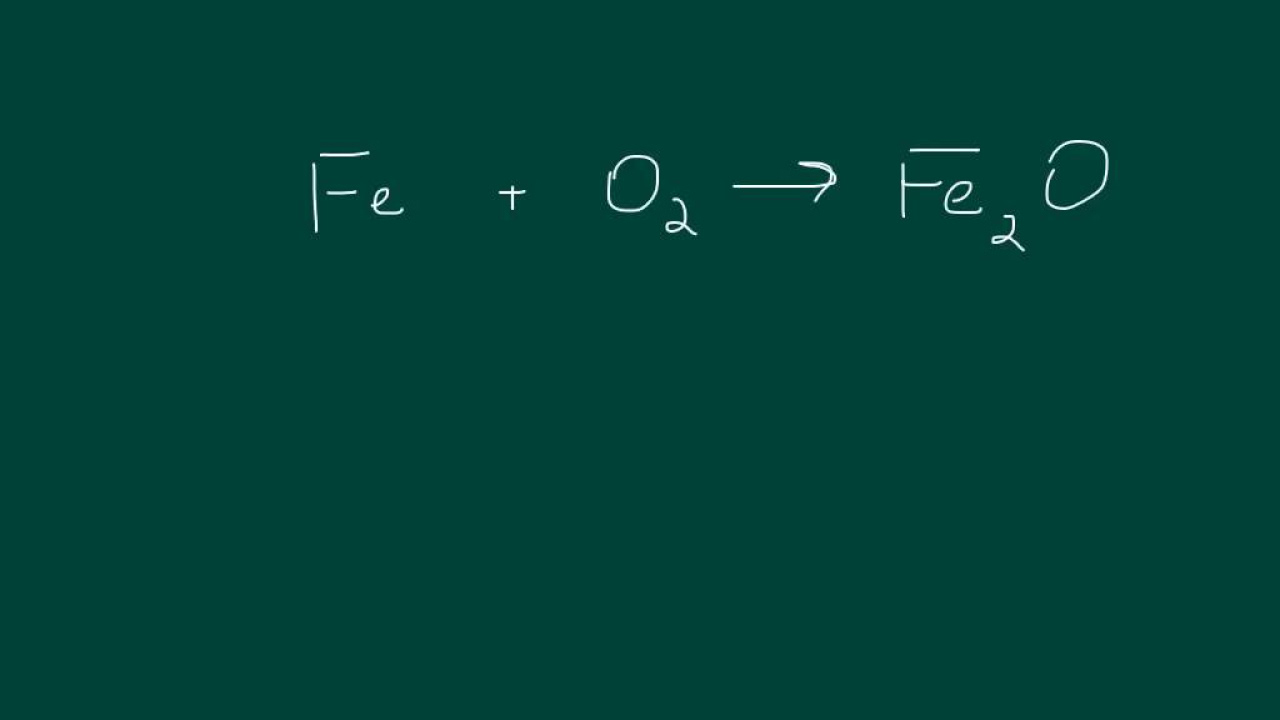How to Balance a Chemical Equation EASY
TLDRThis video offers a straightforward method for balancing chemical equations. It involves dividing the equation, listing elements on both sides, counting them, and adjusting coefficients to ensure equal representation of each element. The process emphasizes balancing metals first, followed by non-metals, and concludes with hydrogen and oxygen.
Takeaways
- 📝 The script explains a method for balancing chemical equations, which involves dividing the equation into reactants and products and listing the elements on each side.
- 🔍 It emphasizes the importance of counting the atoms of each element on both sides of the equation to ensure they are equal after balancing.
- 🛠 The process begins by balancing the metals first, which in the example are iron and potassium, by adjusting the coefficients in front of the compounds.
- ⚔ The metals must be balanced before moving on to non-metals, highlighting the sequential nature of the balancing process.
- 🔄 After metals, the script instructs to balance non-metals, except for hydrogen and oxygen, which are addressed last in the process.
- 📐 The script provides a step-by-step guide to adjusting coefficients, starting with metals, then non-metals, and finally hydrogen and oxygen.
- ✏️ It is recommended to use a pencil when writing coefficients, as they may need to be changed multiple times during the balancing process.
- 🔄 The script illustrates that after changing coefficients for one element, it's necessary to reassess and adjust the coefficients for other elements to maintain balance.
- 🔢 The process involves multiplication of the coefficients to reflect changes in the number of atoms for each element in the compounds.
- 💡 The script points out that often hydrogen and oxygen will balance themselves out naturally when following the outlined steps.
- 🎯 The final step is to double-check that all elements, including metals, non-metals, hydrogen, and oxygen, are balanced in the equation.
- 📚 The video concludes by summarizing the method as simple and quick for balancing chemical equations, with the example provided demonstrating its effectiveness.
Q & A
What is the main topic of the video?
-The main topic of the video is how to balance chemical equations using a quick and easy method.
What is the first step in balancing a chemical equation according to the video?
-The first step is to divide the equation into reactant and product sides and list all the different elements on each side.
What elements are listed on the reactant side in the example provided?
-The elements listed on the reactant side are iron (Fe), sulfur, oxygen, potassium, and hydrogen.
How many oxygen atoms are initially counted on the reactant side?
-Initially, there are 13 oxygen atoms on the reactant side (4 oxygens inside the brackets multiplied by 3, plus an additional oxygen).
What is the goal of balancing chemical equations?
-The goal of balancing chemical equations is to ensure that the number of each element is the same on both the reactant and product sides.
What is the order in which the elements are balanced in the video?
-The order is first metals, then non-metals (except hydrogen and oxygen), followed by hydrogen, and finally oxygen.
Why can't subscripts in chemical compounds be changed during the balancing process?
-Subscripts in chemical compounds represent the fixed number of atoms in a molecule and cannot be changed as they define the compound's identity.
What is the purpose of changing coefficients in the balancing process?
-Changing coefficients is done to adjust the number of molecules of each compound so that the number of atoms of each element is equal on both sides of the equation.
How does the video demonstrate balancing the metals in the equation?
-The video demonstrates balancing the metals by adjusting the coefficients in front of the compounds containing iron and potassium to match their quantities on both sides.
What is the final step in the balancing process shown in the video?
-The final step is to ensure that all elements, including hydrogen and oxygen, are balanced, resulting in a completely balanced chemical equation.
Why is it recommended to use a pencil when adding coefficients in the balancing process?
-Using a pencil is recommended because the coefficients might need to be adjusted multiple times during the balancing process, and pencil allows for easy corrections.
Outlines
🔍 Balancing Chemical Equations: Initial Steps
This paragraph introduces the process of balancing chemical equations using a systematic method applicable to any chemical reaction. The speaker begins by dividing the equation into reactants and products and lists all the different elements on each side. The elements are then counted to determine their quantities in the reactants and products. The goal is to equalize the number of atoms for each element on both sides of the equation. The paragraph outlines the steps to achieve balance: first, balancing metals, then non-metals except hydrogen and oxygen, and finally, balancing hydrogen and oxygen last. The speaker emphasizes the importance of using coefficients to adjust the number of molecules without altering the subscripts within the chemical formulas.
🧪 Adjusting Coefficients to Balance Equations
The second paragraph delves into the practical application of balancing the given chemical equation by adjusting coefficients. The speaker starts by balancing the metals, iron and potassium, by ensuring an equal number of atoms on both sides of the equation. This involves changing coefficients in front of the chemical compounds to match the number of metal atoms. The process continues with adjusting coefficients for non-metals, such as sulfur, and then for hydrogen and oxygen. The speaker highlights the importance of using a pencil for this task, as coefficients may need to be revised as the equation is balanced. The paragraph illustrates the iterative nature of balancing chemical equations, where changes in one part of the equation necessitate re-evaluation of other parts to maintain balance. The final step confirms that all elements are balanced, resulting in a correctly balanced chemical equation.
Mindmap
Keywords
💡Chemical Equations
💡Reactants
💡Products
💡Elements
💡Balancing
💡Metals
💡Non-metals
💡Coefficients
💡Hydrogen
💡Oxygen
💡Conservation of Mass
Highlights
Introduction to a quick and easy method for balancing chemical equations.
Drawing a line to divide reactant and product sides of the equation.
Listing all different elements on each side of the equation.
Counting the elements on the reactant side, including 2 irons, 3 sulfurs, 1 potassium, 1 hydrogen, and 13 oxygens.
Counting the elements on the product side, including 1 iron, 2 potassiums, 1 sulfur, 3 hydrogens, and 7 oxygens.
The goal of balancing is to equalize the number of each element on both sides.
Step-by-step process to balance chemical equations, starting with metals.
Balancing iron by adjusting coefficients, resulting in 2 irons on both sides.
Adjusting coefficients for potassium to balance the metal elements.
Balancing non-metals, except for hydrogen and oxygen, by adjusting coefficients.
Balancing sulfur by tripling the coefficients where sulfur is present.
Rechecking and adjusting the balance of metals after modifying non-metal coefficients.
Balancing hydrogen by adjusting coefficients where hydrogen is present.
Balancing oxygen by adjusting coefficients and ensuring the total count matches on both sides.
Finalizing the balanced chemical equation with the correct coefficients.
Emphasizing the importance of using a pencil for adjustments due to the iterative nature of balancing.
Observation that hydrogen and oxygen often balance themselves when following the outlined steps.
Conclusion highlighting the simplicity and effectiveness of the method for balancing chemical equations.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: