16. Thermodynamics: Gibbs Free Energy and Entropy
TLDRThis lecture delves into the concept of spontaneous change in chemical reactions, emphasizing that spontaneity is not solely determined by enthalpy (delta H) but also by entropy (delta S) and temperature, as described by Gibbs free energy (delta G). The instructor uses everyday examples like rust formation and ice melting to illustrate these principles, demonstrating how reactions can be spontaneous even if they are endothermic. The importance of understanding delta G is highlighted through calculations and a hands-on demonstration involving hydrogen peroxide, showing how entropy can drive a reaction to be spontaneous despite a positive enthalpy change.
Takeaways
- 📚 The lecture discusses the concept of spontaneous change in chemical reactions, emphasizing that spontaneity is not solely dependent on external factors like heat but can occur naturally.
- 🔍 Spontaneous reactions proceed in the forward direction without external intervention and can be influenced by factors such as temperature and pressure.
- 🌡 The script provides examples of spontaneous reactions, including the formation of rust (iron plus oxygen), which is an exothermic process with a negative delta H.
- 🔄 The hydrolysis of ATP to ADP is highlighted as another spontaneous process, important in biological systems for energy storage and release, with a negative delta H.
- ❄️ The melting of ice is presented as an endothermic process with a positive delta H, yet it is still spontaneous, illustrating that enthalpy is not the sole determinant of spontaneity.
- 📉 The key to understanding spontaneity lies in Gibbs free energy (delta G), which takes into account both enthalpy (delta H) and entropy (delta S) changes.
- ⚖️ The sign of delta G indicates whether a reaction is spontaneous (negative), at equilibrium (zero), or non-spontaneous (positive) under constant temperature and pressure conditions.
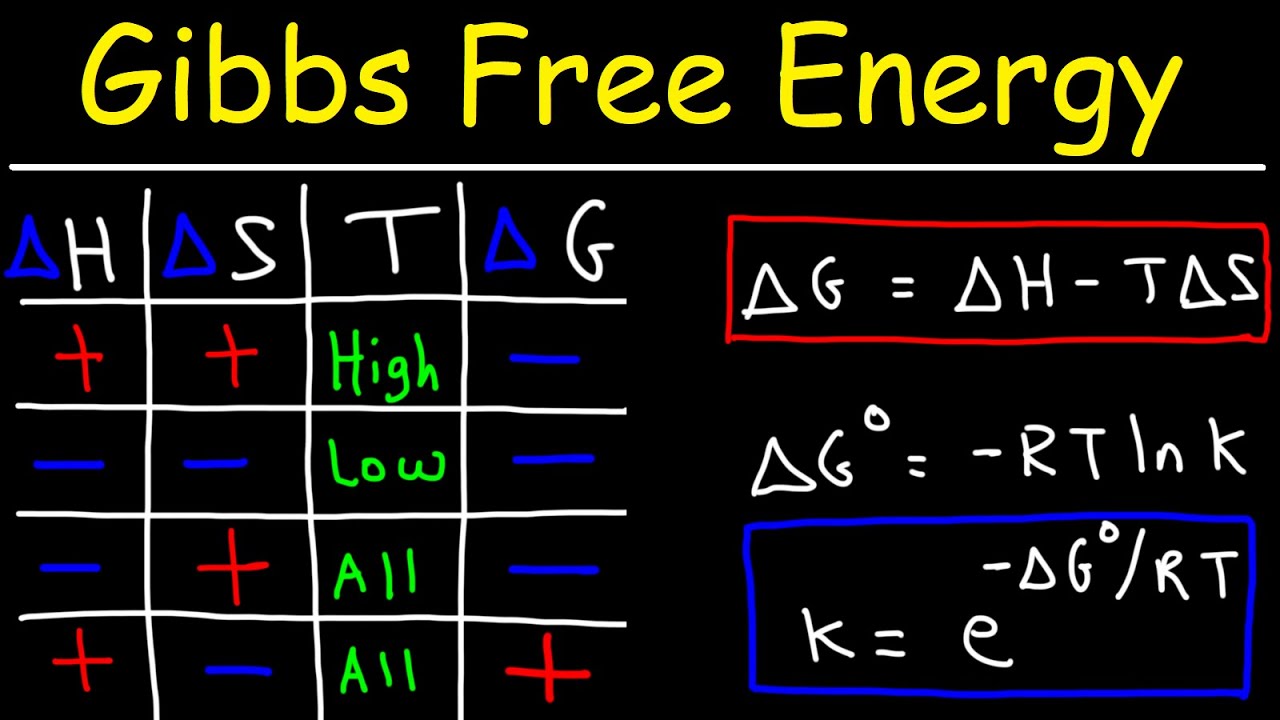
- 🔢 The calculation of delta G involves the use of the equation delta G = delta H - T delta S, where T is the temperature in Kelvin, and delta S is the change in entropy.
- 🔄 Entropy (delta S) is a measure of disorder in a system, with positive values indicating an increase in disorder and negative values indicating a decrease.
- 🧪 A demonstration using hydrogen peroxide illustrates the increase in entropy and the spontaneity of the reaction, even though it is endothermic, by producing oxygen gas that inflates soap bubbles.
- 🌡 The temperature's role in spontaneity is underscored, with the script explaining that reactions with negative delta H and positive delta S are spontaneous at all temperatures.
Q & A
What is a spontaneous change in the context of chemical reactions?
-A spontaneous change is a reaction that proceeds in the forward direction without any outside intervention, such as the addition of heat.
Why is the rusting of iron considered a spontaneous process?
-Rusting is a spontaneous process because it occurs naturally without any external influence, such as heat, and is a reaction between iron and oxygen.
What does it mean for a reaction to be exothermic?
-An exothermic reaction is one that releases heat to the surroundings, indicated by a negative delta H value.
What is ATP hydrolysis and why is it a spontaneous process?
-ATP hydrolysis is the reaction where ATP (adenosine triphosphate) reacts with water to form ADP (adenosine diphosphate) and inorganic phosphate. It is a spontaneous process due to the release of energy stored in the phosphate bonds, with a negative delta H0 value.
How does the melting of ice or snow illustrate the concept of spontaneity in physical changes?
-The melting of ice or snow is a spontaneous process because it transitions from a solid to a liquid state without the need for external energy input, illustrating the natural tendency towards increased disorder or entropy.
What role does Gibbs free energy (delta G) play in determining the spontaneity of a reaction?
-Gibbs free energy (delta G) is a thermodynamic potential that predicts whether a reaction will proceed spontaneously. A negative delta G indicates a spontaneous reaction, while a positive delta G indicates a non-spontaneous reaction at constant temperature and pressure.
What is the significance of the equation delta G = delta H - T delta S in understanding reaction spontaneity?
-The equation delta G = delta H - T delta S is crucial in determining the spontaneity of a reaction by considering both the enthalpy change (delta H) and the entropy change (T delta S). It shows that spontaneity is influenced by both heat exchange and disorder in the system.
Why is the conversion of joules to kilojoules necessary when calculating delta G?
-The conversion of joules to kilojoules is necessary to maintain unit consistency, as most thermodynamic values are expressed in kilojoules, especially when dealing with molar quantities.
What does a positive delta S value signify in terms of entropy change?
-A positive delta S value signifies an increase in the disorder of the system, indicating that the entropy has increased due to factors such as greater molecular freedom or a phase change from solid to liquid or gas.
How does the concept of entropy relate to the stability of a substance?
-Entropy, as a measure of disorder, is related to the stability of a substance in that substances with higher entropy (greater disorder) are generally more stable. This is because they have more internal degrees of freedom, such as in the case of gases compared to solids.
What is the significance of the demo involving hydrogen peroxide, soap, and yeast?
-The demo with hydrogen peroxide, soap, and yeast serves as a visual representation of the spontaneous decomposition of hydrogen peroxide into water and oxygen gas, illustrating the increase in entropy and the concept of a spontaneous reaction.
Why is the melting of ice at room temperature considered a spontaneous endothermic process?
-The melting of ice at room temperature is considered a spontaneous endothermic process because, despite the positive delta H0 (absorbing heat), the increase in entropy (positive delta S) results in a negative delta G0, indicating spontaneity.
What can the standard Gibbs free energy of formation (delta Gf°) values be used for?
-The standard Gibbs free energy of formation (delta Gf°) values can be used to determine the thermodynamic stability of a compound relative to its constituent elements in their standard states. A negative delta Gf° indicates that the compound is stable, while a positive value suggests instability.
How does the concept of thermodynamics differ from kinetics in the context of chemical reactions?
-Thermodynamics provides information about the stability and spontaneity of a reaction, indicating whether a reaction is energetically favorable. Kinetics, on the other hand, deals with the rates of reactions, determining how quickly or slowly a reaction will proceed, regardless of its thermodynamic favorability.
Outlines
🔍 Introduction to Spontaneous Change and Gibbs Free Energy
The script introduces the concept of spontaneous change in chemical reactions, which occurs without external influence. It discusses how temperature and pressure can affect spontaneity, using the example of rust formation, an exothermic process. The script then transitions to the role of Gibbs free energy (delta G) as a determinant of spontaneity, which depends on both enthalpy (delta H) and entropy (delta S). The importance of the equation delta G = delta H - T delta S is highlighted as a predictor for spontaneous reactions.
📚 Calculating Gibbs Free Energy and Understanding Spontaneity
This section delves into the calculation of Gibbs free energy (delta G) using the equation delta G = delta H - T delta S, emphasizing the significance of temperature and entropy in determining the spontaneity of a reaction. It provides an example calculation with positive delta H and positive delta S, resulting in a spontaneous reaction despite the endothermic nature of delta H. The importance of unit conversion from joules to kilojoules is also discussed.
🌡️ The Impact of Temperature on Spontaneity and Entropy
The paragraph explores how temperature affects the spontaneity of reactions, particularly when delta H and delta S have opposite signs. It explains that reactions with negative delta H and positive delta S are spontaneous at all temperatures. The concept of entropy as a measure of disorder is introduced, with examples from New England stone walls and the speaker's dog Shep, illustrating the natural tendency towards increased disorder.
🧪 Demonstrating Entropy Increase with a Hydrogen Peroxide Reaction
A practical demonstration of entropy increase is described, where hydrogen peroxide decomposes into water and oxygen gas, visualized through soap bubbles. The reaction is catalyzed by yeast and includes a discussion on the calculation of entropy change (delta S) for the reaction, resulting in a positive value indicative of increased disorder.
🔄 Discussing the Role of Entropy in Reaction Spontaneity
This section discusses how entropy changes (delta S) can drive the spontaneity of reactions. It explains the calculation of delta S for reactions using standard entropy values of reactants and products. The melting of ice is given as an example of an endothermic process that is spontaneous due to an increase in entropy, highlighting the importance of considering both enthalpy and entropy in determining spontaneity.
📉 Free Energy of Formation and its Implications for Stability
The concept of free energy of formation (delta G sub f) is introduced, which is analogous to enthalpy of formation but includes entropy considerations. The script explains how delta G of formation values can indicate the stability of a compound relative to its elements. A negative delta G of formation signifies that a compound is stable and spontaneous in its forward formation reaction but non-spontaneous in the reverse direction, as exemplified by the formation of carbon dioxide.
🛠️ Thermodynamics and Kinetics: Understanding Stability and Reaction Rates
The final paragraph distinguishes between thermodynamics, which determines the stability of compounds, and kinetics, which deals with reaction rates. It reiterates the importance of both fields in understanding chemical reactions and the limitations of thermodynamics in predicting reaction speeds. The script concludes with a mention of the continued use of the equation delta G = delta H - T delta S in subsequent lessons.
Mindmap
Keywords
💡Spontaneous Change
💡Gibbs Free Energy (ΔG)
💡Enthalpy (ΔH)
💡Entropy (ΔS)
💡Exothermic Reaction
💡Endothermic Reaction
💡ATP Hydrolysis
💡Equilibrium
💡Significant Figures
💡Catalyst
💡State Function
Highlights
MIT OpenCourseWare provides high-quality educational resources for free.
Spontaneous change is a reaction that proceeds without outside intervention.
The temperature can affect whether a reaction is spontaneous.
Rust is an example of a spontaneous process.
ATP hydrolysis to ADP is a spontaneous process with a negative delta H0.
Glucose oxidation in the body stores energy in ATP, which is vital for energy release.
The melting of ice is an endothermic process that is spontaneous.
Ammonium nitrate decomposes spontaneously with a positive delta H.
Gibbs free energy (delta G) is the key to predicting spontaneity, not just delta H.
Delta G is influenced by both enthalpy (delta H) and entropy (delta S).
A negative delta G indicates a spontaneous reaction.
Equilibrium is represented by delta G equals 0.
The importance of converting units when calculating delta G.
A positive delta H and positive delta S can still result in a spontaneous reaction.
Glucose oxidation to CO2 and water is a spontaneous but slow reaction at room temperature.
Entropy is a measure of disorder, with positive delta S indicating increased disorder.
The concept of entropy is illustrated with stone walls in New England.
Phase changes, such as from solid to liquid, can be used to predict changes in entropy.
The demonstration of hydrogen peroxide decomposition shows entropy increase.
Entropy of reactions can be calculated using absolute entropy values.
The relationship between delta G, delta H, and delta S is crucial for understanding reaction spontaneity.
The stability of CO2 compared to its elements is discussed in terms of delta G of formation.
Thermodynamics and kinetics are both essential for explaining reactions.
Transcripts
Browse More Related Video

6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant | Organic Chemistry

Entropy: Embrace the Chaos! Crash Course Chemistry #20
Gibbs Free Energy - Entropy, Enthalpy & Equilibrium Constant K

Endergonic, exergonic, exothermic, and endothermic reactions | Khan Academy

Gibbs free energy and spontaneity | Chemistry | Khan Academy

AP Chem - Unit 9 Review - Applications of Thermodynamics in 10 Minutes - 2023
5.0 / 5 (0 votes)
Thanks for rating: