6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant | Organic Chemistry
TLDRThe video script delves into the fundamental concepts of thermodynamics as they pertain to organic chemistry, focusing on the intricate relationship between entropy, Gibbs free energy, and the equilibrium constant. It clarifies that while entropy is often colloquially associated with disorder, it is mathematically defined as the reversible heat transfer divided by temperature. The script also emphasizes the second law of thermodynamics, which states that the entropy of the universe increases for any spontaneous process. The role of Gibbs free energy in determining whether a reaction is spontaneous is explored, with a negative delta G indicating a spontaneous process and a positive delta G indicating a non-spontaneous one. The interplay between enthalpy (delta H) and entropy (delta S) in predicting spontaneity at various temperatures is discussed, highlighting that while enthalpy often dominates, entropy becomes more significant at higher temperatures. The video concludes by examining the link between standard Gibbs free energy (delta G standard) and the equilibrium constant (K), illustrating that a reaction favoring products corresponds to a negative delta G standard and a K value greater than one, whereas a reaction favoring reactants is associated with a positive delta G standard and a K value less than one.
Takeaways
- 🔄 **Entropy and Disorder**: Entropy is often equated with disorder, with more disorder correlating to higher entropy. It's technically defined as the reversible heat transfer divided by temperature, and also related to the number of microstates in a system.
- ⚙️ **Second Law of Thermodynamics**: For any spontaneous process in the universe, the total entropy (ΔS) must increase, meaning ΔS for the universe is always positive.
- 🌡️ **Entropy and Temperature**: Entropy (ΔS) can be affected by temperature, with higher temperatures potentially increasing the impact of ΔS on the Gibbs free energy equation.
- 🌬️ **Gas Molecules and Entropy**: An increase in the number of gas molecules typically results in higher entropy due to greater molecular motion and dispersion, leading to more microstates.
- 🧬 **Molecules and Phases**: More molecules in the same phase generally lead to higher entropy. This is particularly relevant for organic chemistry reactions often taking place in solution.
- ⚡ **Gibbs Free Energy (ΔG)**: ΔG represents the energy available to do work in a system. A negative ΔG indicates a spontaneous process, while a positive ΔG means the process is non-spontaneous without external energy input.
- 🔥 **Enthalpy (ΔH) and Spontaneity**: Enthalpy often dominates the Gibbs free energy equation. A negative ΔH usually, but not always, indicates a spontaneous reaction.
- ↔️ **Equilibrium Constant (K_eq)**: At chemical equilibrium, the ratio of products to reactants is represented by K_eq. It's a key factor in determining the spontaneity of a reaction at equilibrium.
- 🔄 **ΔG, ΔH, and ΔS Relationship**: The equation ΔG = ΔH - TΔS is central to determining spontaneity. At higher temperatures, the TΔS term can become more significant, affecting whether a reaction is spontaneous.
- ✅ **Spontaneity Conditions**: A reaction is spontaneous when ΔH is negative (exothermic) and ΔS is positive, at all temperatures. Conversely, a positive ΔH and negative ΔS make a reaction non-spontaneous at all temperatures.
- 🔄 **Entropic Favorability**: Reactions with positive ΔS become more spontaneous at higher temperatures, an important concept in organic chemistry.
Q & A
What is the relationship between entropy and disorder in a system?
-Entropy is often simplified as a measure of disorder, though they are not technically the same. An increase in disorder generally correlates with an increase in entropy, and vice versa. Entropy is more precisely defined as the reversible element of heat transfer divided by the temperature in Kelvin, or in terms of the number of microstates in a system.
What does the second law of thermodynamics state about entropy?
-The second law of thermodynamics states that for a spontaneous process, the change in entropy (ΔS) of the entire universe will be positive, meaning there is an overall increase in entropy for any spontaneous process in the universe.
How does the number of gas molecules affect entropy?
-The more gas molecules there are in a system, the higher the entropy. This is because gas molecules are more spread out and move faster than those in a liquid or solid state, allowing for more microstates and thus greater disorder.
What is Gibbs free energy and why is it important in determining spontaneity of a reaction?
-Gibbs free energy (ΔG) is the energy available to do work in a system. A negative ΔG indicates that a reaction is spontaneous, as it means the system is using up energy that was available for work. Conversely, a positive ΔG means the reaction is non-spontaneous and requires an input of energy from the surroundings.
What are the terms 'exergonic' and 'endergonic' in relation to Gibbs free energy?
-Exergonic reactions are those where ΔG is negative, indicating a spontaneous process that releases energy. Endergonic reactions have a positive ΔG, meaning they require an input of energy to proceed and do not occur spontaneously.
How does the entropy change (ΔS) influence the spontaneity of a reaction at high temperatures?
-At high temperatures, the term involving ΔS in the equation ΔG = ΔH - TΔS becomes more significant. If ΔS is positive, the reaction becomes more spontaneous as temperature increases because the positive contribution of the TΔS term outweighs the ΔH term when multiplied by a high temperature.
What is the relationship between the standard Gibbs free energy change (ΔG°) and the equilibrium constant (Keq)?
-The standard Gibbs free energy change (ΔG°) is related to the equilibrium constant (Keq) by the equation ΔG° = -RT ln(Keq), where R is the gas constant and T is the temperature in Kelvin. A positive Keq corresponds to a negative ΔG°, favoring the formation of products at equilibrium.
What happens to the spontaneity of a reaction when ΔH is positive and ΔS is negative?
-When ΔH is positive and ΔS is negative, the reaction is non-spontaneous at all temperatures. The positive ΔH and negative ΔS contribute to a positive ΔG, which indicates that the reaction does not proceed spontaneously under any conditions.
How does the spontaneity of a reaction change with temperature when ΔH is negative and ΔS is positive?
-When ΔH is negative and ΔS is positive, the reaction is spontaneous at all temperatures. The negative ΔH and the positive ΔS both contribute to a negative ΔG, regardless of the temperature.
What is the implication of a reaction having a positive ΔH and a positive ΔS?
-A reaction with a positive ΔH and a positive ΔS can be spontaneous, but only at high temperatures. The positive ΔS term, when multiplied by a high temperature, can outweigh the positive ΔH, resulting in a negative ΔG and thus a spontaneous reaction.
What does it mean if the equilibrium constant (Keq) is exactly 1 for a reaction?
-If the equilibrium constant (Keq) is exactly 1, it means that at equilibrium, there are equal amounts of reactants and products present. This does not necessarily mean the reaction is non-spontaneous; it simply indicates that the reaction is equally likely to proceed in the forward or reverse direction at equilibrium.
Outlines
🔄 Understanding Entropy and its Relation to Reaction Spontaneity
The first paragraph introduces the concept of entropy, which is often simplified as a measure of disorder but is more technically defined as the reversible heat transfer divided by temperature in Kelvin. It discusses the correlation between increased disorder and increased entropy, as well as the factors affecting entropy such as the number of gas molecules and the overall number of molecules in a system. The paragraph also touches on the second law of thermodynamics, which states that the entropy of the universe increases for spontaneous processes. It concludes with the impact of entropy on predicting the spontaneity of reactions in organic chemistry.
🌡️ Gibbs Free Energy and its Role in Determining Spontaneity
The second paragraph delves into Gibbs free energy, which is the energy available to do work. It explains that a negative change in Gibbs free energy (ΔG) indicates a spontaneous process, while a positive ΔG suggests a non-spontaneous one. The paragraph also introduces the terms exergonic and endergonic to describe reactions with negative and positive ΔG, respectively. It further discusses the interplay between ΔG, ΔH (enthalpy change), and ΔS (entropy change), emphasizing that at higher temperatures, the entropy term becomes more significant in determining spontaneity. A table is used to illustrate the conditions under which a reaction is spontaneous based on the signs of ΔH and ΔS.
⚙️ The Universe's Preference for Lower Energy and Higher Disorder
This paragraph explores the conditions under which reactions are non-spontaneous, highlighting that the universe favors reactions that lower enthalpy and increase disorder. It explains that if a reaction does not meet these criteria, it will not proceed spontaneously. The discussion also covers scenarios where the universe is given only one of the two preferred conditions, leading to conditional spontaneity based on temperature. The relationship between ΔG, ΔH, and ΔS is further elaborated upon, showing how the magnitude of these values and the temperature can influence whether a reaction is spontaneous.
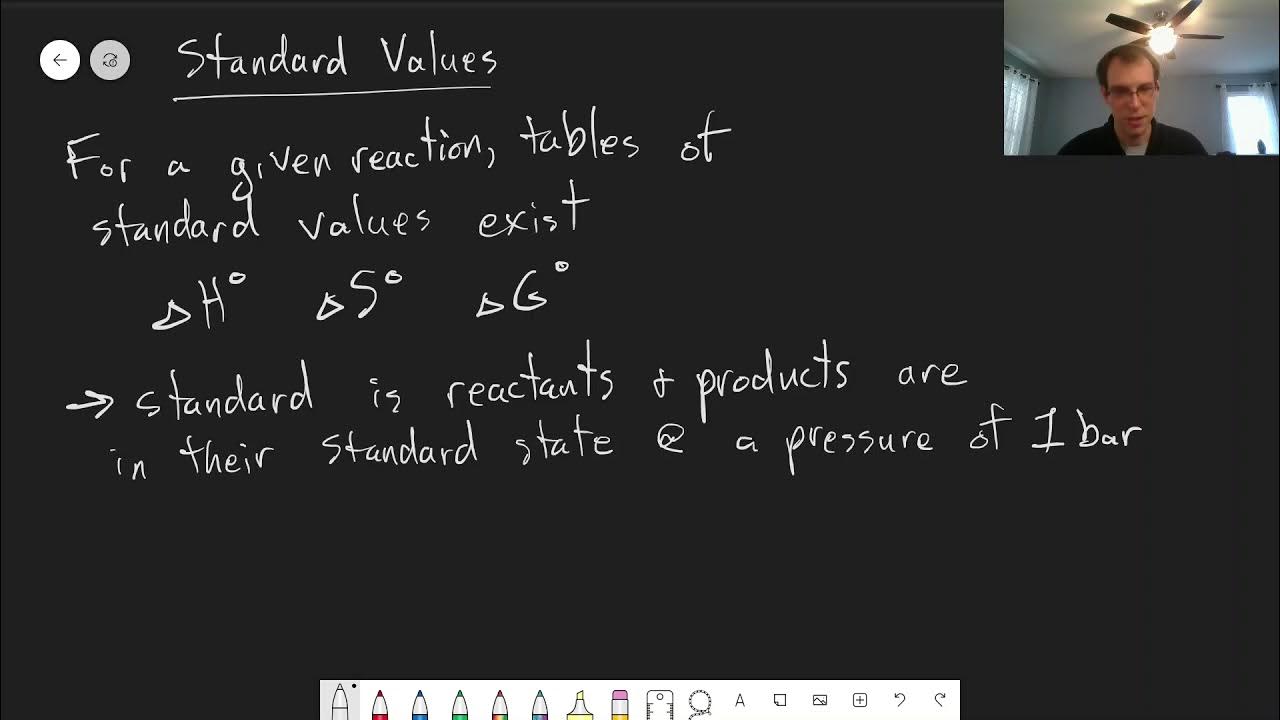
🔗 Linking ΔG° with Equilibrium Constants in Organic Reactions
The final paragraph establishes the relationship between the standard change in Gibbs free energy (ΔG°) and the equilibrium constant (K_eq) of a reaction. It explains that the natural logarithm of the equilibrium constant is directly related to ΔG°, and that this relationship can be used to predict whether a reaction favors the formation of products or reactants at equilibrium. The paragraph clarifies that a ΔG° of zero corresponds to an equilibrium constant of one, while a K_eq greater than one indicates a negative ΔG° favoring product formation, and vice versa for a K_eq less than one. This section reinforces the importance of understanding these relationships in the context of organic chemistry.
Mindmap
Keywords
💡Entropy
💡Gibbs Free Energy
💡Equilibrium Constant
💡Thermodynamics
💡Second Law of Thermodynamics
💡Endothermic and Exothermic
💡Exergonic and Endergonic
💡Microstates
💡Spontaneity
💡Enthalpy
💡Temperature
Highlights
Entropy is related to disorder, though not technically the same, with more disorder correlating to higher entropy.
Entropy has a mathematical definition as the reversible element of heat transfer divided by temperature in Kelvin.
Microstates represent the number of different states a system can exist in, influencing entropy.
The second law of thermodynamics states that the entropy of the universe increases for any spontaneous process.
Factors affecting entropy include the number of gas molecules and the increase in the number of molecules in general.
Gibbs free energy is the energy available to do work, with spontaneous processes having a negative delta G.
Delta G is negative for exergonic reactions and positive for endergonic reactions.
The relationship between delta G, delta H, and delta S is given by the equation delta G = delta H - T * delta S.
At elevated temperatures, delta S can have a more significant impact on delta G.
A reaction is spontaneous when delta H is negative and delta S is positive at all temperatures.
If delta H is positive and delta S is negative, the reaction is non-spontaneous at all temperatures.
Reactions with a positive delta H and negative delta S can be spontaneous at low temperatures.
Reactions with a positive delta H and positive delta S are spontaneous at high temperatures.
Entropically favorable reactions become more spontaneous as temperature increases.
The relationship between delta G standard and the equilibrium constant is given by delta G standard = -RT * ln(K_eq).
If K_eq is greater than one, delta G standard is negative, favoring the formation of products.
If K_eq is less than one, delta G standard is positive, favoring the reactants at equilibrium.
Transcripts
Browse More Related Video

16. Thermodynamics: Gibbs Free Energy and Entropy
Chapter 6: K and Standard Gibbs Energy | CHM 214 | 051

Entropy: Embrace the Chaos! Crash Course Chemistry #20

AP Chem - Unit 9 Review - Applications of Thermodynamics in 10 Minutes - 2023

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry
More rigorous Gibbs free energy / spontaneity relationship | Chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: