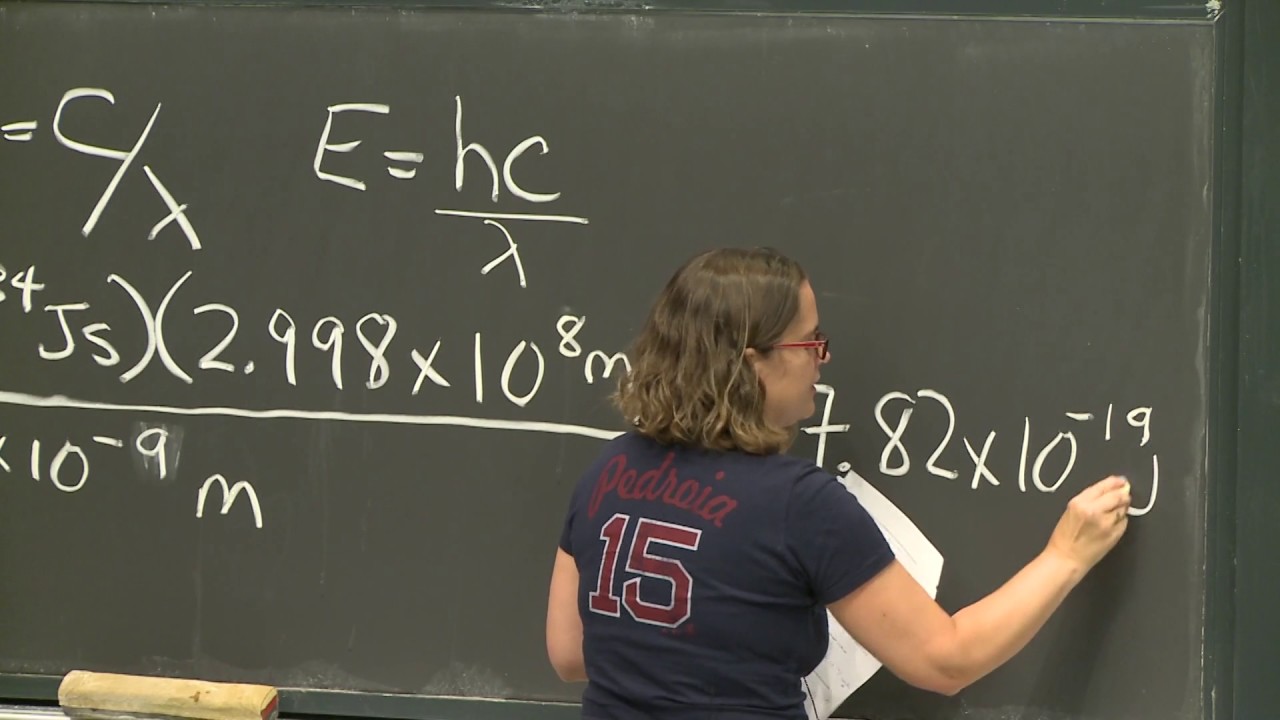3. Wave-Particle Duality of Light
TLDRThis script from an MIT OpenCourseWare lecture delves into the dual wave-particle nature of light, focusing on the photoelectric effect. Professor Catherine Drennan guides students through the historical discovery of subatomic particles, the quantization of energy, and Planck's constant. She illustrates how light's energy, dependent on frequency, leads to electron ejection from metals when photons exceed the work function. The lecture also creatively employs the 'Roy G. Biv' mnemonic for the visible light spectrum and highlights practical applications, including diffraction in x-ray crystallography.
Takeaways
- 📚 The lecture discusses the importance of supporting MIT OpenCourseWare for free educational resources and mentions the website ocw.mit.edu for donations and materials.
- 🎓 Catherine Drennan engages the audience with a clicker question, emphasizing the importance of active participation and reviewing problem set one.
- 🏆 The lecture includes an interactive element where students are rewarded with prizes like an American Chemical Society pen and a LEGO girl chemist for correct answers.
- 🔬 The class revisits the discovery of the electron and the nucleus, highlighting the concept that atoms are mostly empty space with a small, concentrated nucleus.
- 🌊 Drennan uses the analogy of waves, such as those found at the beach, to introduce the periodic nature of waves, including amplitude, wavelength, frequency, and period.
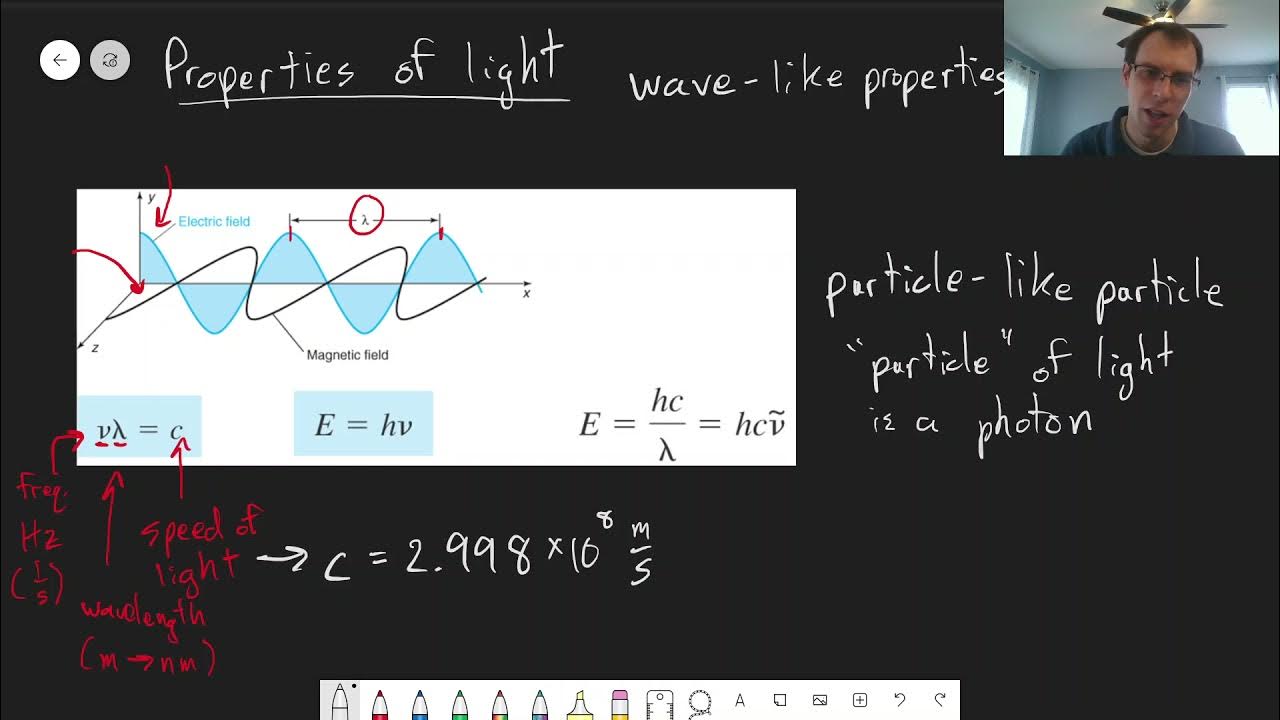
- 🌈 The lecture explains the relationship between the speed of light, wavelength, and frequency, and introduces the concept of wave-particle duality, leading into the photoelectric effect.
- 🎼 A catchy song, Roy G. Biv, is used to help students remember the order of visible light wavelengths from red to violet.
- 🔧 Practical applications of wave interference, such as in acoustics for concert halls and classrooms, and in noise-canceling headphones, are discussed.
- 🧬 The use of constructive and destructive interference in x-ray crystallography to determine the structures of molecules like proteins and nucleic acids is explained.
- ⚛️ Einstein's examination of the photoelectric effect and his realization that light has particle-like properties, quantized in energy packets called photons, is highlighted.
- 🔑 The photoelectric effect experiments showed that the kinetic energy of ejected electrons is related to the frequency of the incoming light, leading to the equation KE = e*(h*ν - φ), where KE is kinetic energy, e is the charge of an electron, h is Planck's constant, ν is frequency, and φ is the work function.
- 💡 The lecture concludes with a demonstration of the photoelectric effect and a reminder of the importance of understanding the relationship between light intensity, photon energy, and the photoelectric effect.
Q & A
What is the purpose of MIT OpenCourseWare and how can one support it?
-MIT OpenCourseWare aims to offer high-quality educational resources for free. Support can be provided through donations or by visiting ocw.mit.edu to view additional materials from hundreds of MIT courses.
What did the audience member explain about finding the limiting reactant?
-The audience member explained that the limiting reactant is found by dividing the amount of moles for both substances by their respective molar coefficients and identifying which one is limiting (in this case, O). Then, by using the molar fraction, which is one Al2O3 for every 3FeO, the result is multiplied by 12 to get the final answer.
What is the significance of the discovery of the electron and the nucleus in understanding atomic structure?
-The discovery of the electron and the nucleus was significant because it revealed that atoms are mostly empty space with a small, concentrated nucleus that can deflect alpha particles. This understanding changed the way scientists thought about matter and led to the realization that atoms have subatomic particles.
What is wave-particle duality and why is it important in understanding light?
-Wave-particle duality is the concept that light, and matter, can exhibit both wave-like and particle-like properties. This duality is important for understanding light because it helps explain various phenomena observed in experiments, such as interference patterns and the photoelectric effect.
What are the characteristics of waves discussed in the script?
-The characteristics of waves discussed include amplitude, wavelength (denoted by lambda), frequency (denoted by nu), period, and intensity. These characteristics define the behavior of waves such as water waves, sound waves, and light waves.
What is the relationship between the speed of light, wavelength, and frequency?
-The speed of light is equal to the product of the wavelength and the frequency. This relationship is expressed by the equation c = λν, where c is the speed of light, λ is the wavelength, and ν is the frequency.
How does the color of light relate to its wavelength and frequency?
-The color of light is related to its wavelength and frequency such that colors with longer wavelengths (e.g., red) have lower frequencies, while colors with shorter wavelengths (e.g., violet) have higher frequencies.
What practical applications are mentioned for the principles of constructive and destructive interference?
-Practical applications of constructive and destructive interference include the design of symphony halls and classrooms for better acoustics, noise-canceling headphones, and the determination of molecular structures using x-rays in crystallography.
What is the photoelectric effect and why was it significant in understanding light's particle-like properties?
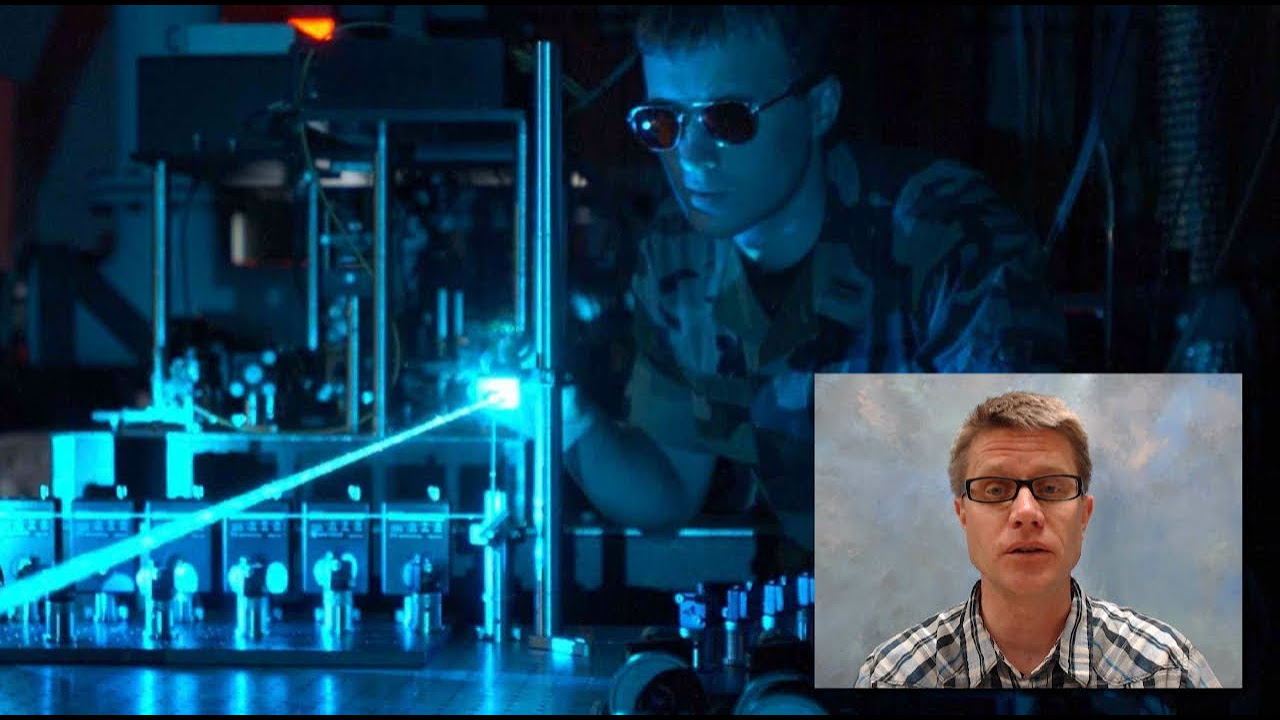
-The photoelectric effect is the emission of electrons from a metal surface when it is exposed to light (specifically, UV light) of a certain frequency. It was significant because it demonstrated light's particle-like properties, showing that light can be thought of as consisting of packets of energy, or photons, which have an energy proportional to their frequency.
How did Einstein's explanation of the photoelectric effect contribute to the understanding of energy and frequency?
-Einstein explained the photoelectric effect by stating that the energy of light is proportional to its frequency, with the proportionality constant being Planck's constant (h). This led to the equation E = hν, where E is the energy of the photon, h is Planck's constant, and ν is the frequency. This understanding revolutionized the concept of energy quantization.
What is the relationship between the intensity of light and the number of ejected electrons in the photoelectric effect?
-The intensity of light, which is the number of photons hitting per second, is directly proportional to the number of ejected electrons. If the photons have enough energy to overcome the work function of the metal, increasing the intensity will result in more electrons being ejected.
Outlines
📚 Introduction to MIT OpenCourseWare and Classroom Interaction
The script begins with an introduction to MIT OpenCourseWare, emphasizing its commitment to providing free, high-quality educational resources under a Creative Commons license. Viewers are encouraged to support MIT OpenCourseWare through donations and to explore additional course materials on their website. The scene transitions to a classroom setting where Professor Catherine Drennan engages students in a clicker question exercise, highlighting the importance of student participation and the value of practice. The summary also touches on the rewarding aspect of correctly answering questions, as evidenced by the distribution of an American Chemical Society pen as a prize.
🌪 Discussing Limiting Reactants and the Wave-Particle Duality
This paragraph delves into a chemistry lesson where students determine the limiting reactant by dividing the moles of substances by their respective molar coefficients. The discussion serves as a refresher on a previous problem set and introduces the concept of wave-particle duality, setting the stage for an exploration of light's characteristics as both a wave and a particle. The narrative includes a light-hearted moment where the promise of a better prize later in the class is humorously juxtaposed with the current prize, an ACS pen.
🌊 Waves and Their Fundamental Properties
The focus shifts to the characteristics of waves, starting with a review of basic wave properties like amplitude, wavelength, frequency, and period. The paragraph uses the analogy of water waves at Revere Beach to explain these concepts, drawing parallels with sound waves and electromagnetic radiation. It also introduces the concept of wave intensity and how these properties can be applied to different types of waves, including light waves. The summary underscores the importance of understanding periodic behavior and the quantitative aspects of wave dynamics.
🔵🌈 The Electromagnetic Spectrum and the Speed of Light
This section discusses the electromagnetic spectrum, highlighting the relationship between wavelength and frequency, and how they are inversely related due to the constant speed of light. It explains the concept using the mnemonic 'ROY G. BIV' to remember the order of visible light colors and their corresponding wavelengths. The paragraph also emphasizes the significance of the speed of light (c), its constant nature, and its role in various wave phenomena, including the relationship between wavelength and frequency.
🎵 A Musical Interlude for Wavelength Memorization
The script introduces a creative method for memorizing the order of wavelengths using a catchy song by They Might Be Giants, which helps students remember the sequence of colors in the electromagnetic spectrum. The summary captures the light-hearted approach to learning, illustrating how even young children can benefit from such mnemonic devices, and shares a personal anecdote about the impact of the song on Professor Drennan's daughter.
🌈 Beyond Visible Light: The Electromagnetic Spectrum
The paragraph expands on the electromagnetic spectrum beyond visible light, discussing the properties and applications of various types of waves such as radio waves, microwaves, infrared, ultraviolet, x-rays, and gamma rays. It humorously touches on the practical advice of using the popcorn button in microwaves and the importance of sunscreen to protect against UV rays. The summary provides a comprehensive overview of the different regions of the electromagnetic spectrum and their unique characteristics.
🌐 Wave Interference and Its Practical Applications
This section explores the concept of wave interference, including constructive and destructive interference, and their practical applications in various fields such as acoustics in symphony halls and classrooms, and in the design of noise-canceling headphones. The paragraph also mentions the role of interference in the research of Professor Drennan, particularly in the use of x-rays to determine the structures of molecules. The summary highlights the significance of interference in both theoretical understanding and real-world applications.
🔬 The Photoelectric Effect and Light as a Particle
The script delves into the photoelectric effect, which demonstrates the particle-like properties of light. It describes the experimental observations where ultraviolet light ejects electrons from metal surfaces, and how this phenomenon could only be explained by considering light as consisting of quantized energy packets, or photons. The summary explains the relationship between the frequency of the incoming light, the threshold frequency, and the kinetic energy of the ejected electrons, leading to the introduction of Planck's constant.
⚡ Einstein's Explanation of the Photoelectric Effect
This paragraph discusses Einstein's examination of the photoelectric effect and his formulation of the relationship between the kinetic energy of ejected electrons and the frequency of incoming light. It explains how Einstein's analysis led to the understanding that light consists of photons, each with energy proportional to its frequency, described by the equation E=hf. The summary captures the significance of this discovery in revolutionizing the concept of energy and its quantization.
🔍 The Implications of Photon Energy and Intensity
The final paragraph examines the implications of Einstein's photoelectric effect equations, particularly focusing on how the energy of photons and the intensity of light affect the ejection of electrons from metal surfaces. It clarifies the misconceptions about the relationship between light intensity and the kinetic energy of electrons, and how the number of ejected electrons is related to the intensity of the light. The summary provides a clear explanation of the photoelectric effect's principles and their significance in understanding the quantum nature of light.
Mindmap
Keywords
💡Creative Commons license
💡MIT OpenCourseWare
💡Limiting reactant
💡Molar coefficient
💡Wave-particle duality
💡Photoelectric effect
💡Amplitude
💡Wavelength
💡Frequency
💡Intensity
💡Constructive and destructive interference
💡Synchrotron
💡Crystallography
💡Planck's constant
💡Threshold frequency
💡Work function
Highlights
MIT OpenCourseWare offers high-quality educational resources for free, supported by donations.
82% of students answered the clicker question correctly, indicating a good understanding of the material.
The concept of limiting reactant is introduced, essential for solving stoichiometry problems in chemistry.
The importance of practicing chemical problem-solving, especially for complex topics like the wave-particle duality of light.
Historical context of the discovery of the electron and nucleus, highlighting the atom's mostly empty space.
Introduction of the wave-particle duality concept, fundamental in quantum mechanics, explaining both wave-like and particle-like properties of matter and radiation.
Explanation of the photoelectric effect, a key experiment that demonstrated light's particle-like properties.
Review of wave characteristics such as amplitude, wavelength, frequency, period, and intensity, crucial for understanding wave behavior.
The relationship between wavelength, frequency, and the speed of light, with c = λν, a fundamental equation in physics.
The significance of the speed of light being constant, leading to the interdependence of wavelength and frequency.
The use of the ROYGBIV mnemonic to remember the order of visible light wavelengths, a helpful tool for students.
The electromagnetic spectrum's range beyond visible light, including radio waves, microwaves, infrared, ultraviolet, x-rays, and gamma rays.
Practical applications of wave interference in acoustics, such as in designing symphony halls and classrooms, and in noise-canceling headphones.
The role of constructive and destructive interference in determining the structures of molecules using x-rays, a technique vital in modern chemistry and biology.
Einstein's explanation of the photoelectric effect using the concept of photons, which revolutionized the understanding of light's quantized nature.
The equation E=hf relating the energy of a photon to its frequency, a cornerstone of quantum mechanics.
The distinction between the effects of light intensity on the number of ejected electrons versus their kinetic energy in the photoelectric effect.
A demonstration of the photoelectric effect using a clicker question to reinforce the concept and its implications.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: