Chapter 18: Properties of Light | CHM 214 | 150
TLDRThe transcript delves into the fundamental principles of spectrophotometry, emphasizing the dual nature of light as both a wave and a particle. It explains light's properties, including its wavelength (λ), frequency (ν), and the speed of light (c), and how these relate to each other through equations. The concept of a photon as a packet of energy is introduced, with Planck's constant (h) linking the wave and particle descriptions of light. The inverse relationship between energy and wavelength, and the direct relationship between energy and wave number is highlighted, noting the importance of these concepts in understanding spectroscopy and the electromagnetic spectrum.
Takeaways
- 🌟 Spectrophotometry uses light to make analytical measurements of molecules or atoms, relying on understanding light's properties.
- 📈 Light is often described as having wave-like properties, being an electromagnetic wave with oscillating electric and magnetic fields.
- 🌀 The wavelength (lambda) represents the distance between the highest points (peaks) of the wave, and is commonly measured in nanometers for visible light.
- 🔄 The frequency (nu) of light is the number of cycles per second, and when multiplied by the wavelength, it gives the speed of light (c), a fundamental constant of nature.
- 💡 The speed of light is approximately 2.998 × 10^8 meters per second, which is a constant that allows the conversion between frequency and wavelength.
- 🌈 Light exhibits both wave-like and particle-like properties; the particle of light is called a photon, a term coined by Einstein.
- ⚡ The energy of a photon is given by Planck's constant (h) times the frequency of the light, relating the wave and particle descriptions of light.
- 🔢 The equation E = hν relates the energy of light to its frequency, while E = hc/λ shows that energy is inversely related to wavelength.
- 🎵 The wave number (ν̃) is the reciprocal of the wavelength and is directly proportional to the energy of the light, often used in spectroscopy.
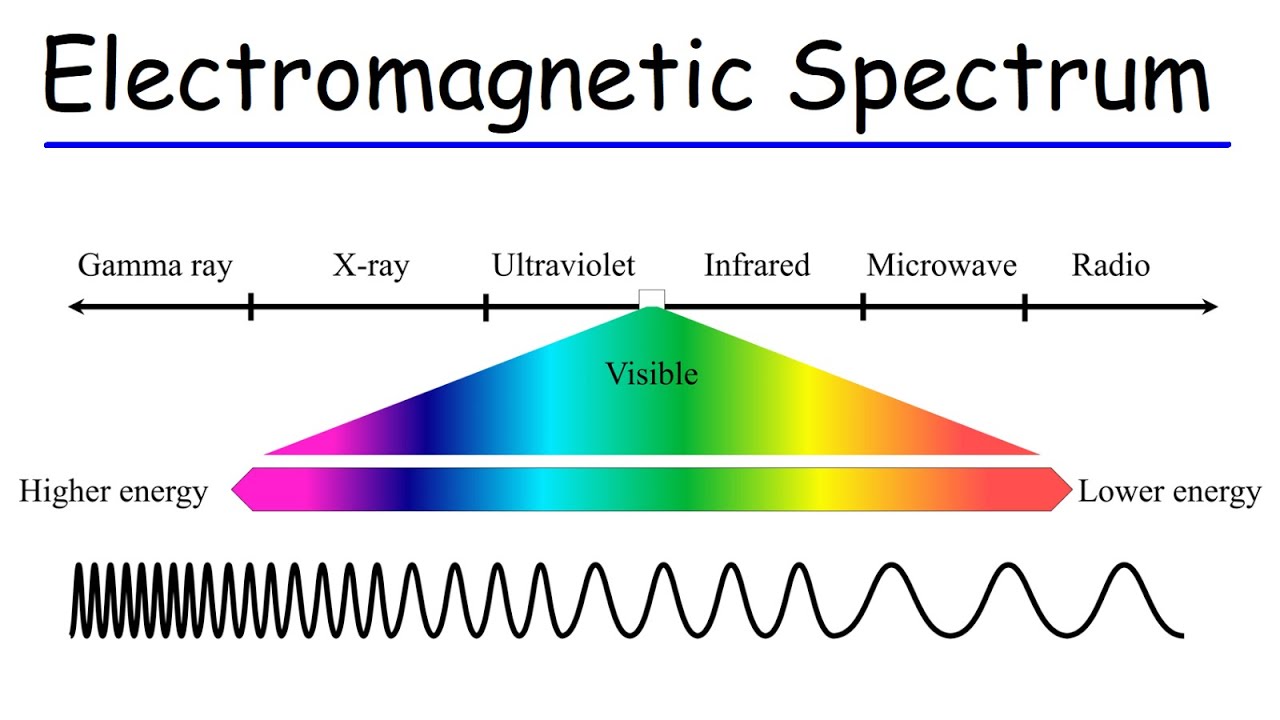
- 🌠 Different regions of the electromagnetic spectrum have varying wavelengths and are used to study different properties of matter.
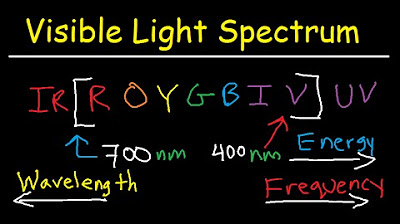
- 📚 Infrared spectroscopists often use wave numbers, while visible light spectroscopists may switch between nanometers and wave numbers depending on the context.
Q & A
What is spectrophotometry and how does it use light?
-Spectrophotometry is a technique that uses light to make analytical measurements of molecules or atoms. It involves analyzing the interaction of light with matter to determine certain properties of the substance being studied.
How is light described in terms of wave-like properties?
-Light is often described as having wave-like properties, meaning it behaves similar to an electromagnetic wave. This is characterized by an electric field oscillating in one plane, perpendicular to which there is a magnetic field oscillating at the same frequency. The self-propagating wave has a particular wavelength, denoted by the symbol lambda.
What is the relationship between wavelength (lambda) and the wave?
-The wavelength (lambda) represents the distance between two consecutive points in the same phase of the wave, such as the highest point (peak) to the next highest point (peak), or from the lowest point (trough) to the next lowest point (trough).
How is the speed of light (c) related to frequency (nu) and wavelength (lambda)?
-The speed of light (c) is a constant and is related to both frequency (nu) and wavelength (lambda) by the equation c = lambda * nu. This equation allows for the conversion between frequency and wavelength, as they are inversely related to each other.
What are the units for frequency and how is it represented?
-Frequency is measured in units of hertz (Hz), which is equivalent to one over second or cycles per second. It represents the number of cycles of the wave passing a given point per second.
What are the different possible wavelengths of light and how are they related to energy?
-Light can have wavelengths ranging from meters (for radio waves) to smaller than nanometers (for X-rays and UV light). The energy of light is directly related to its frequency and inversely related to its wavelength, meaning shorter wavelengths correspond to higher energy.
How is light described as both a wave and a particle?
-Light exhibits both wave-like and particle-like properties, a concept known as wave-particle duality. As a particle, light is described as photons, which are packets of energy.
What is a photon and how is its energy quantified?
-A photon is a particle of light, considered as a packet of energy. The energy of a photon is quantified by Planck's constant (h) times the frequency (nu) of the light, represented by the equation E = h * nu.
What is Planck's constant and its value?
-Planck's constant (h) is a fundamental constant that relates the energy of a photon to its frequency. Its value is approximately 6.626 × 10^-34 joules times seconds (J·s).
How is the wave number related to the energy of light?
-The wave number, represented by the symbol nu with a tilde (~), is directly proportional to the energy of light. It is calculated as the reciprocal of the wavelength (lambda) or as the speed of light (c) divided by the wavelength (c/lambda).
Why do spectroscopists use different units for wavelength and wave number?
-Spectroscopists use different units, such as nanometers for visible light and inverse centimeters for infrared light, because these units can be more convenient for different regions of the electromagnetic spectrum. The choice of unit often depends on the specific application and the properties of matter being studied.
What will be discussed in the next video regarding the electromagnetic spectrum?
-In the next video, the discussion will focus on the different regions of the electromagnetic spectrum and the properties of matter that they address, providing further insights into the interaction of light with various substances.
Outlines
🌟 Understanding Light and Spectrophotometry
This paragraph introduces the fundamental concept of spectrophotometry, which involves using light to make analytical measurements of molecules or atoms. It emphasizes the importance of understanding light, which exhibits wave-like properties and is described as an electromagnetic wave with an electric field oscillating in one plane and a magnetic field oscillating perpendicular to it. The wave's characteristics are defined by its wavelength (lambda), the distance between successive peaks or troughs, and its frequency (nu), which is the number of cycles passing a point per second. These properties are linked by the equation relating frequency and wavelength through the speed of light (c), a fundamental constant of nature. The dual nature of light is also discussed, highlighting its behavior as both a wave and a particle (photon), with the energy of a photon being the product of Planck's constant and the frequency of the light. This relationship is encapsulated in the equation E=hnu, where h is Planck's constant.
🔬 Wave Number and its Relevance in Spectroscopy
The second paragraph delves into the concept of wave number, represented by the symbol nu with a tilde, which is particularly useful in spectroscopy. It explains that wave number is inversely proportional to the wavelength (lambda) and directly proportional to the energy of the light, making it a convenient unit for spectroscopists. The wave number is often used in place of, or alongside, the more commonly known wavelength when analyzing light, especially in different regions of the electromagnetic spectrum. The paragraph also mentions that spectroscopists working with infrared and visible light may switch between using nanometers and wave numbers depending on the context. The discussion sets the stage for the next video, which will explore the various regions of the electromagnetic spectrum and their interaction with matter.
Mindmap
Keywords
💡spectrophotometry
💡light
💡wavelength
💡frequency
💡speed of light
💡photon
💡Planck's constant
💡wave number
💡electromagnetic spectrum
💡energy
Highlights
Fundamental concept of spectrophotometry is using light for analytical measurements of molecules or atoms.
Light is often described as having wave-like properties and is an electromagnetic wave.
White light is represented as an electromagnetic wave with an electric field oscillating and a magnetic field oscillating perpendicular to it.
Wavelength (lambda) is the distance between the highest point of the wave to the highest point again, indicating the wave's size.
Frequency is the number of cycles per second, and when multiplied by wavelength, it gives the speed of the wave (speed of light).
The speed of light (c) is a fundamental constant of nature at 2.998 times 10 to the 8th meters per second.
Light can be described both as a wave with properties of frequency and wavelength and as a particle called a photon.
A photon is a packet of energy, with its amount of energy given by Planck's constant times the frequency of the light.
Planck's constant (h) is 6.626 times 10 to the minus 34 joules times seconds.
The energy of light is directly related to its frequency and inversely related to its wavelength.
The equation E equals hc over lambda relates energy, frequency, and wavelength of light.
Wave number (ν̃) is the inverse of the wavelength and is directly proportional to the energy of the light.
Spectroscopists often use wave numbers, particularly in infrared spectroscopy, as it is convenient for relating energy and wavelength.
Visible light spectroscopists switch between using nanometers and wave numbers depending on the context.
The energy and wave number are directly proportional, while energy and wavelength are inversely proportional.
In the next video, the different regions of the electromagnetic spectrum and their interaction with matter will be explored.
Transcripts
Browse More Related Video

EM waves: wavelength, amplitude, frequency, and Hertz
Electromagnetic Spectrum - Basic Introduction

Photons

Characteristics of Light | Source, Speed, Intensity, Color | Science 7 Quarter 3 Module 4 Week 5
Visible Light Spectrum Explained - Wavelength Range / Color Chart Diagram - Chemistry

Electromagnetic Spectrum Explained - Gamma X rays Microwaves Infrared Radio Waves UV Visble Light
5.0 / 5 (0 votes)
Thanks for rating: